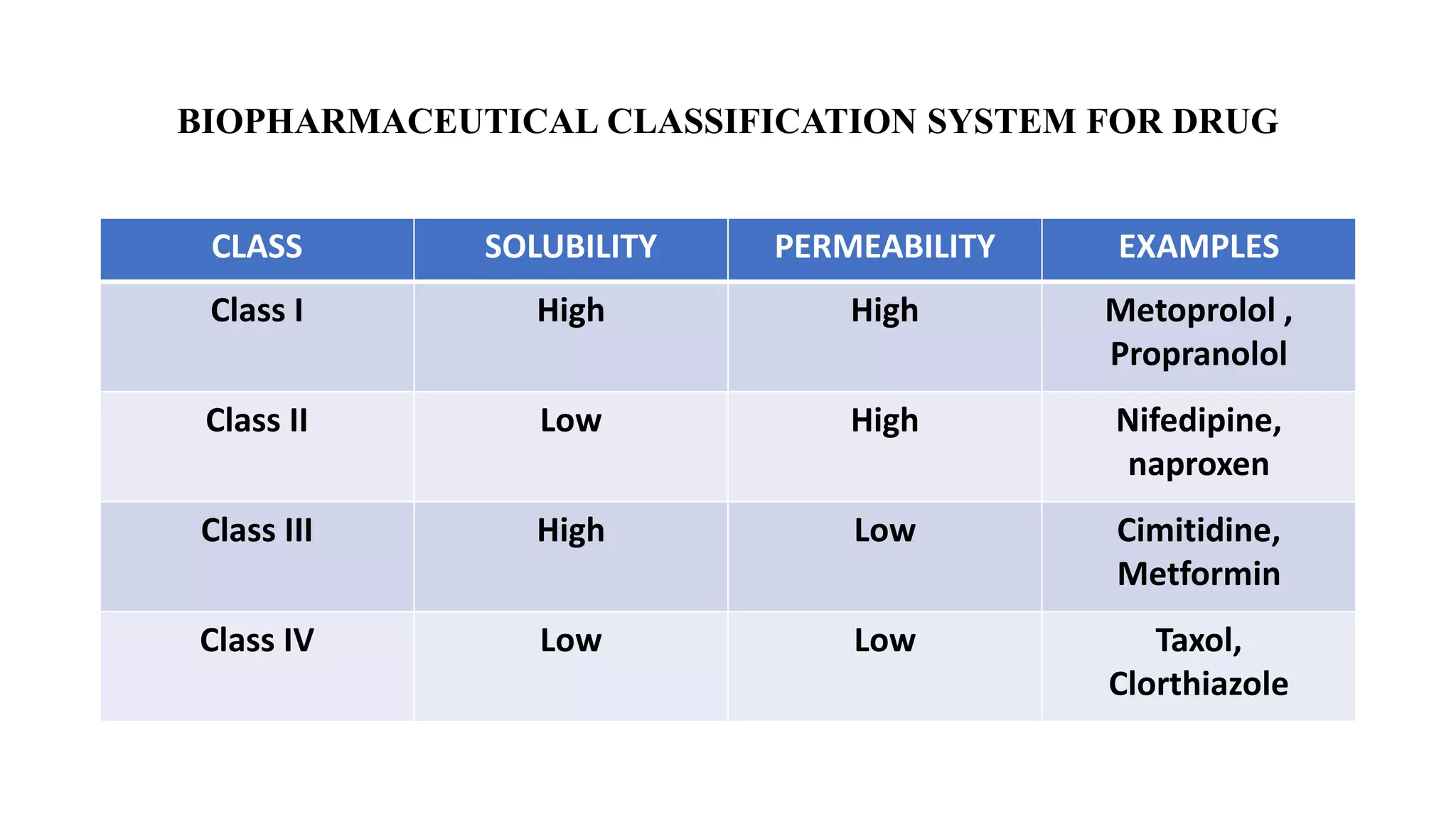
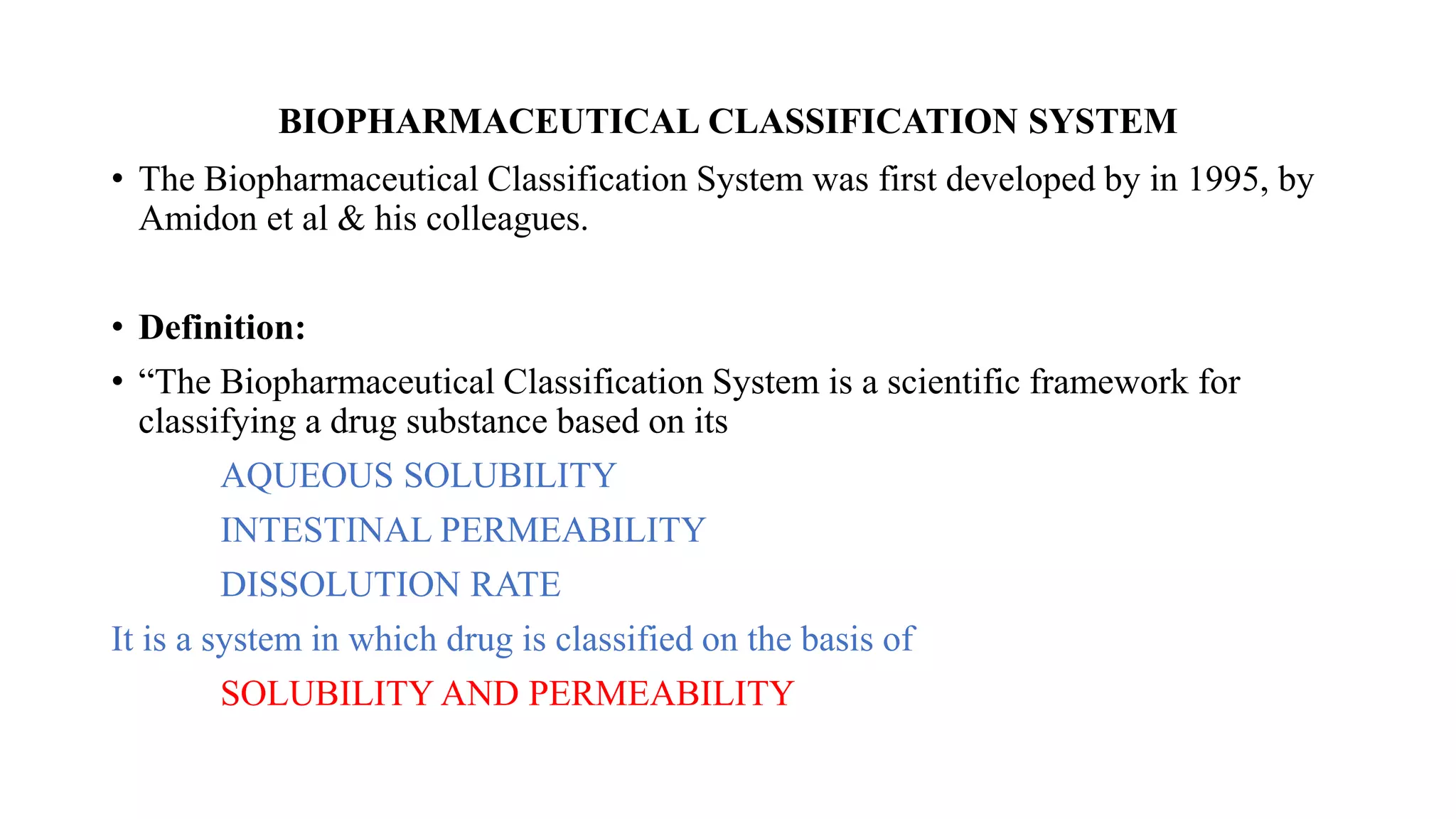
The Biopharmaceutical Classification System (BCS) was developed in 1995 to classify drugs based on their aqueous solubility and intestinal permeability. The BCS classifies drugs into four classes based on being high or low solubility and permeability. Class I drugs are highly soluble and permeable, while Class IV drugs are poorly soluble and permeable. The BCS aims to predict drug performance using solubility and permeability measurements and to determine if in vivo bioequivalence testing is necessary based on in vitro dissolution tests.
![Biopharmaceutical Classification
System [BCS]
By Dr. Manoj Kumar](https://image.slidesharecdn.com/bcsclassifactionofdrug-220102133743/75/Bcs-classification-of-drug-1-2048.jpg)


![DISSOLUTION
• It is process in which solid substance solubilises in given solvent i.e mass transfer
from solid surface to liquid phase.
.
Dissolution Media [900 ml],
1. 0.1 N HCl or simulated gastric fluid (pH 1.2) without enzyme.
pH 4.5 buffer & pH 6.8 buffer,
Simulated intestinal fluid without enzyme.](https://image.slidesharecdn.com/bcsclassifactionofdrug-220102133743/75/Bcs-classification-of-drug-5-2048.jpg)