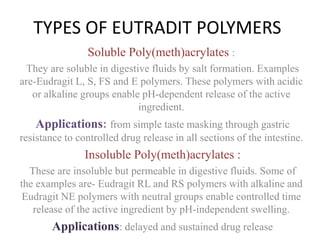
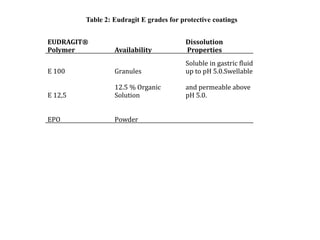
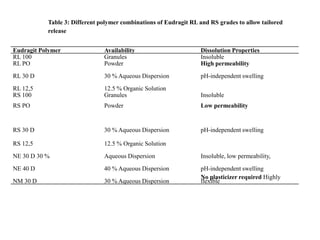
Eudragits are acrylic and methacrylic acid copolymers that are used in pharmaceutical applications for controlled drug release. Different Eudragit polymers allow targeted drug release in various parts of the gastrointestinal tract depending on their pH-dependent solubility. Eudragit polymers can be used to coat formulations for enteric delivery to protect drugs from gastric fluid, or for sustained release in the intestines through diffusion or matrix mechanisms. Common Eudragit grades like Eudragit L, S, and FS are suitable for enteric coatings, while Eudragit RL, RS, and NE polymers are used for sustained release formulations.