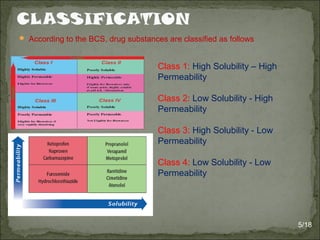
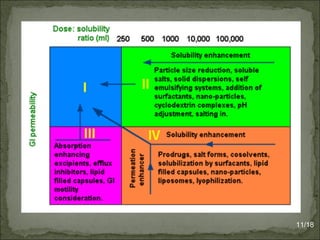
The Biopharmaceutics Classification System (BCS) classifies drug substances into four classes based on solubility and permeability, aiming to streamline drug development and reduce testing requirements. It is widely utilized in areas such as drug formulation, approval processes, and optimizing new chemical entities while allowing biowaivers for class I drugs under specific conditions. Additionally, recent modifications to the classification have introduced more categories based on detailed solubility and permeability assessments to improve prediction of drug absorption.