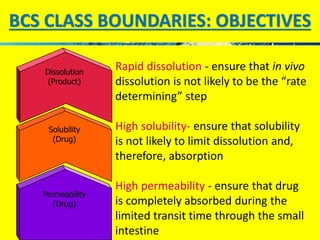
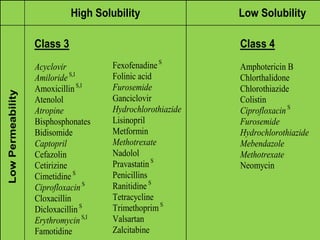
The document discusses the Biopharmaceutical Classification System (BCS), which classifies drug substances based on their aqueous solubility and intestinal permeability. The BCS has four classes based on whether a drug is highly soluble/permeable or low soluble/permeable. It provides a framework to determine if in vitro dissolution tests can replace bioequivalence studies for certain drugs. The BCS considers factors like dose/solubility ratios, dissolution rates, and permeability to classify drugs and determine regulatory applications like biowaivers for bioequivalence studies.









![DISSOLUTION NUMBER
A function of drug release from formulation
• Defined as the ratio of mean residence time to mean
Should exceed 1
dissolution time
Dn= [TGI/TCD]
TGI = Residence time in GI (approx. 180 min)
TCD= Time required for complete dissolution](https://image.slidesharecdn.com/iyznbeegt9mzk3j7jljv-signature-276d8e8396aef2bf62ec45bb63930229d1eb18280d89273ed4789ac2d4914de0-poli-141126051842-conversion-gate01/85/Biopharmaceutics-classification-system-10-320.jpg)