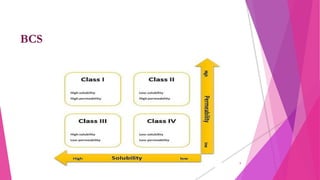
The document discusses the Biopharmaceutical Classification System (BCS), which classifies drug substances based on their aqueous solubility and intestinal permeability. The BCS categorizes drugs into four classes and can be used to guide formulation strategies. It provides a framework for biowaivers where in vivo bioequivalence studies are not required for highly soluble, highly permeable Class I drugs and highly soluble Class III drugs, if the drug products dissolve rapidly. The BCS aims to improve drug development efficiency by identifying bioequivalence tests that can be waived.













![Dissolution
It is process in which solid substance solubilises in given solvent i.e mass
transfer from solid surface to liquid phase
Using USP apparatus I at 100 rpm or USP apparatus II at 50 rpm
Dissolution Media [900 ml]
0.1 N HCl or simulated gastric fluid (pH 1.2) without enzyme
pH 4.5 buffer & pH 6.8 buffer
Simulated intestinal fluid without enzyme
15](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210621121856/85/Biopharmaceutical-classification-system-15-320.jpg)




















