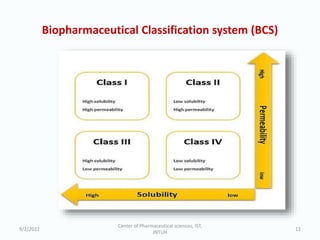
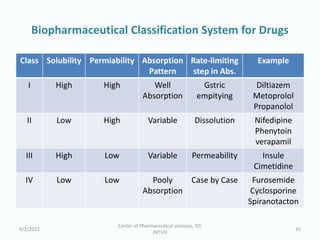
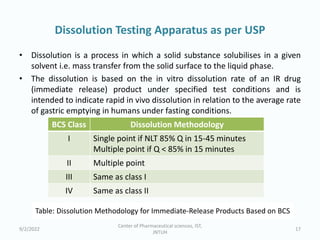
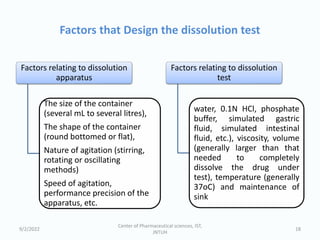
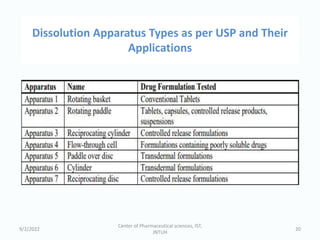
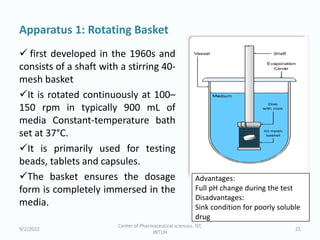
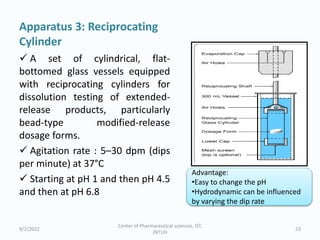
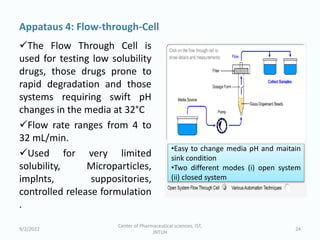
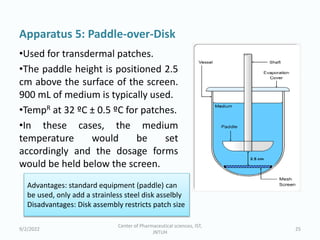
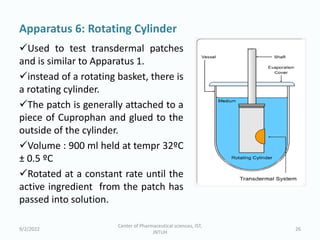
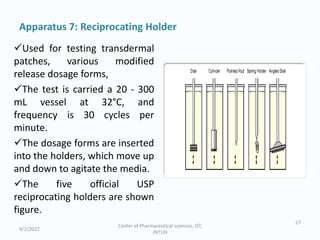
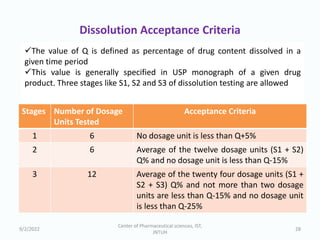
The seminar on the Biopharmaceutical Classification System (BCS) discussed its guidelines for evaluating drug solubility and dissolution, emphasizing its role in drug development and bioequivalence testing. It classified drugs into four categories based on their solubility, permeability, and absorption patterns, providing examples and methodologies for each class. The importance of dissolution testing for ensuring quality and stability in drug products was also highlighted.