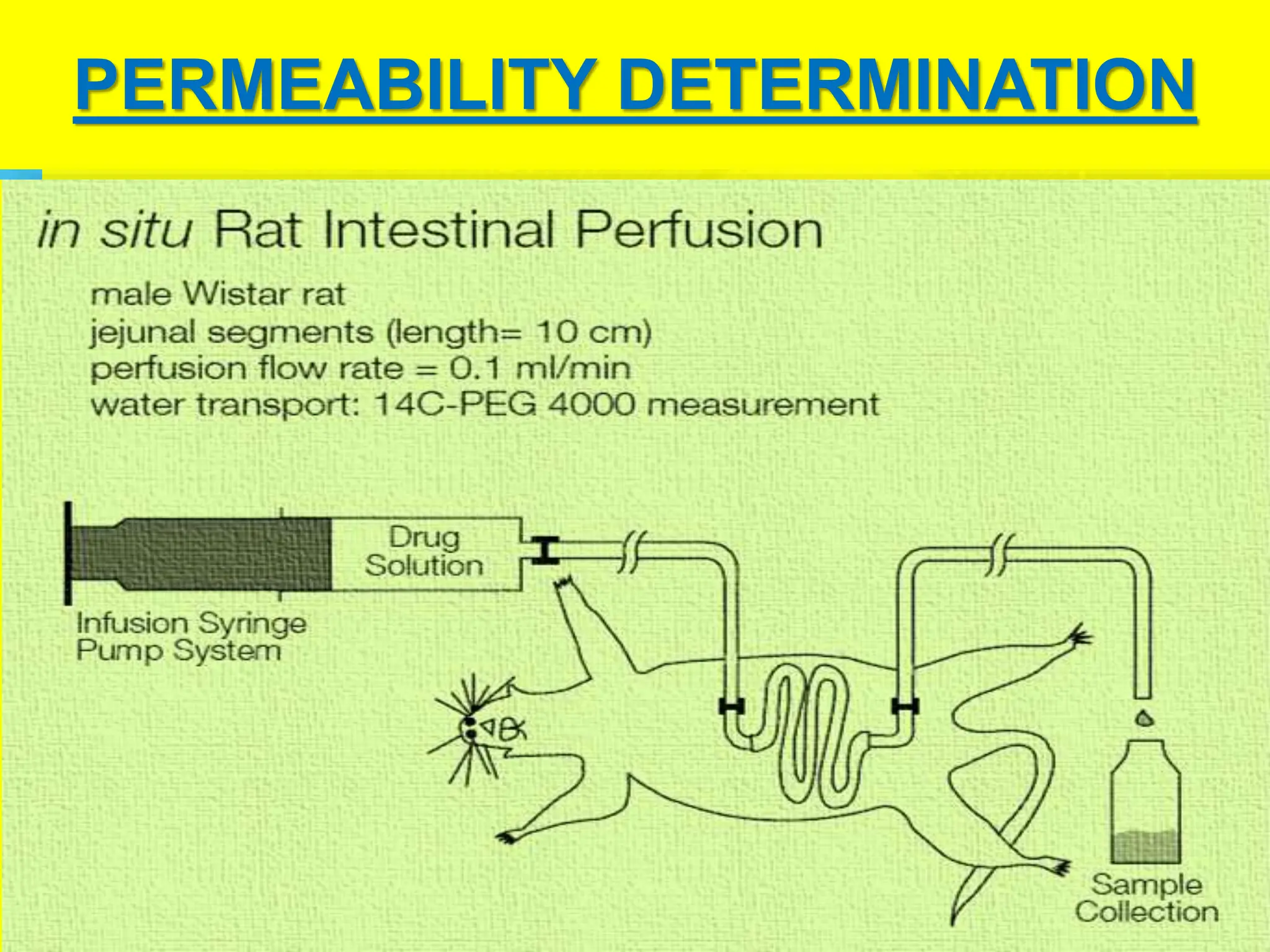
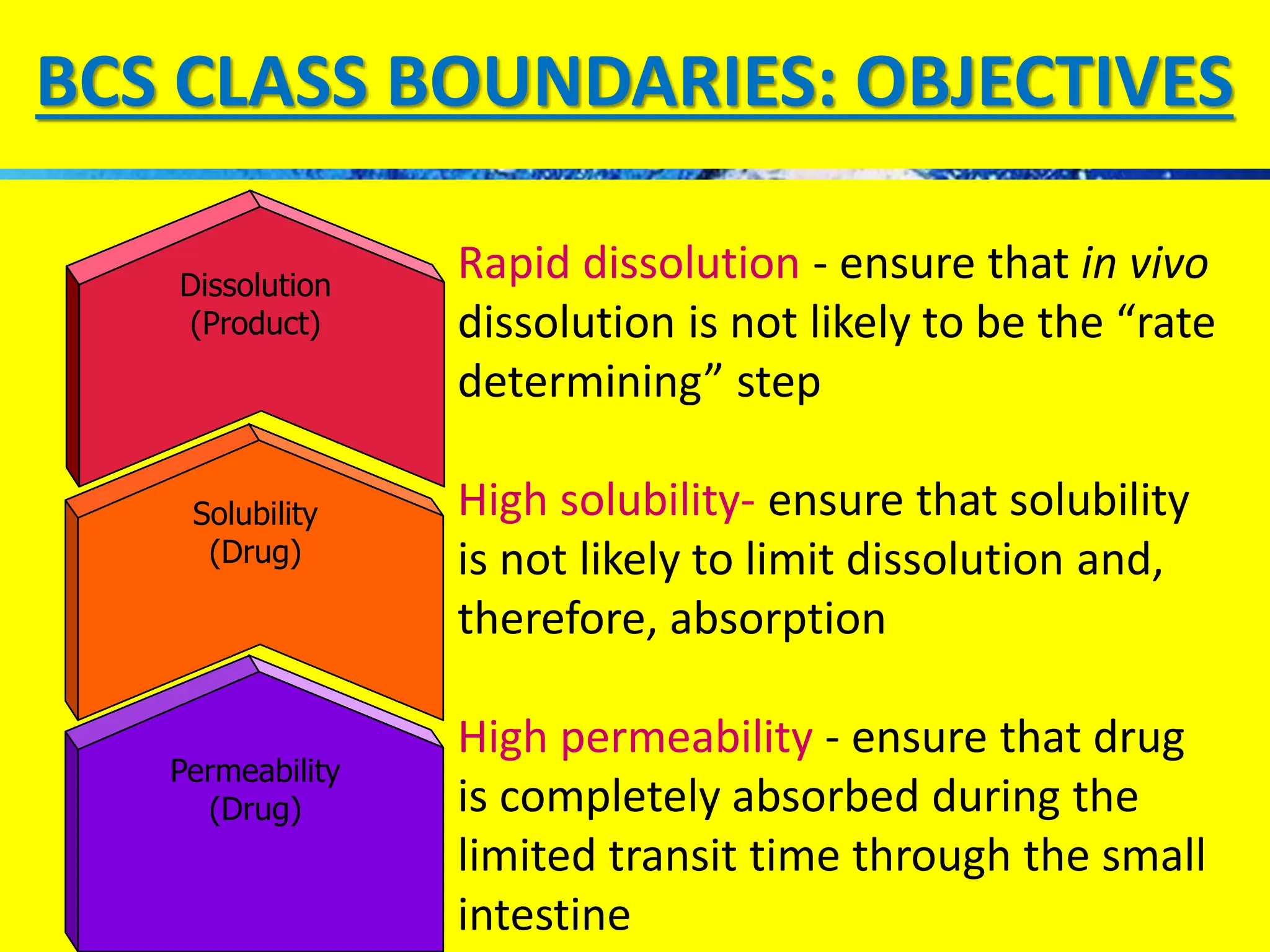
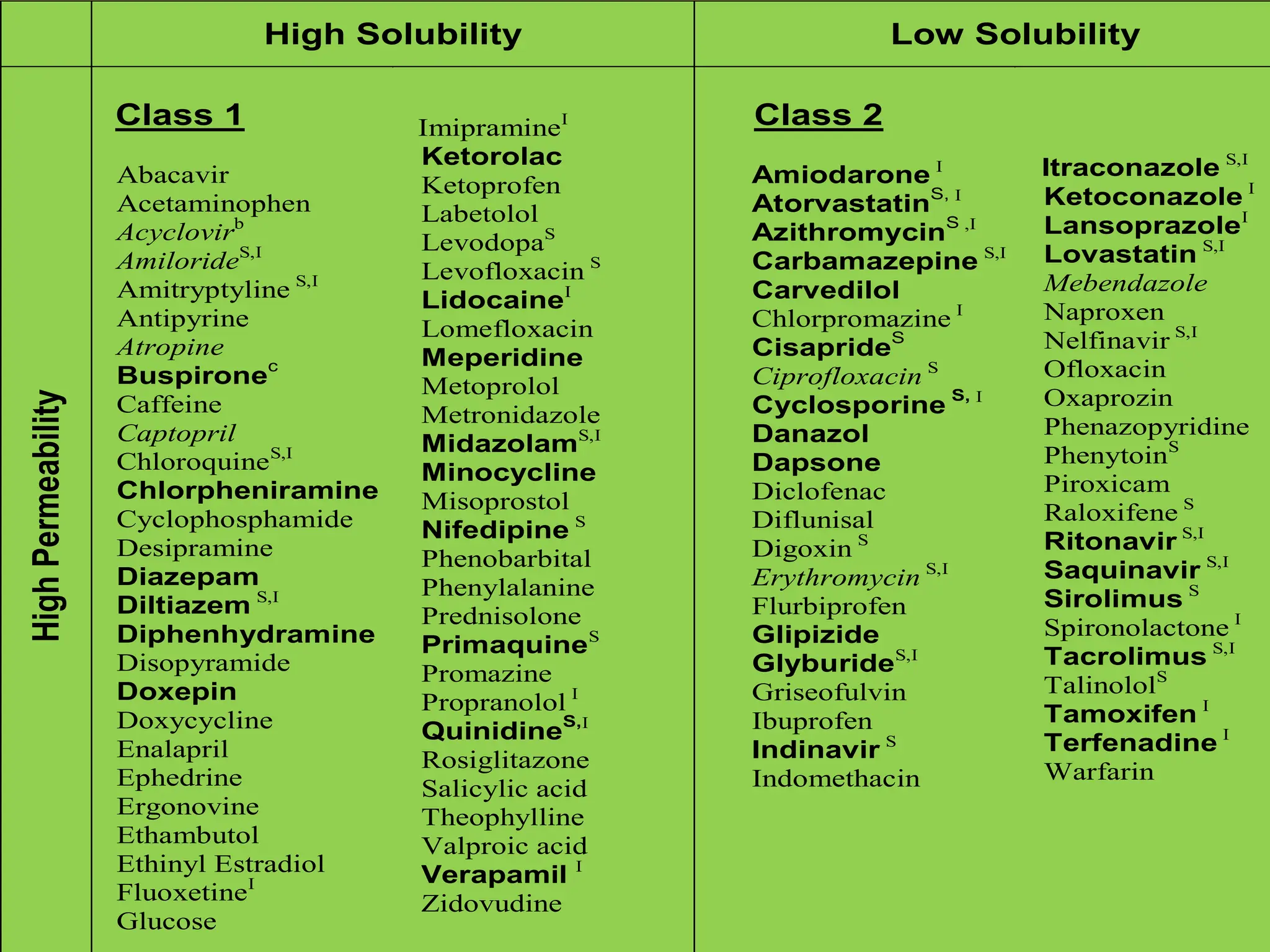
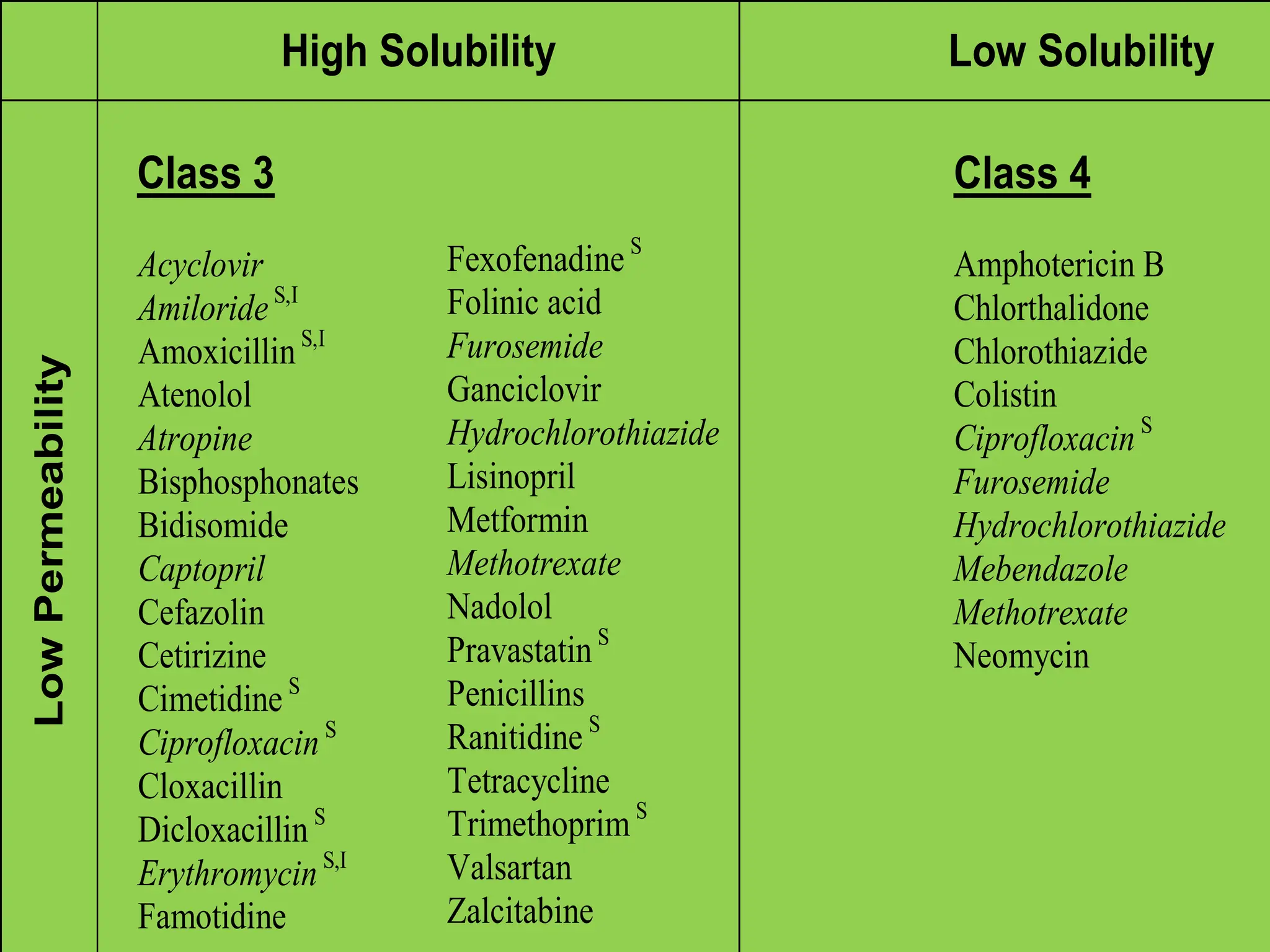
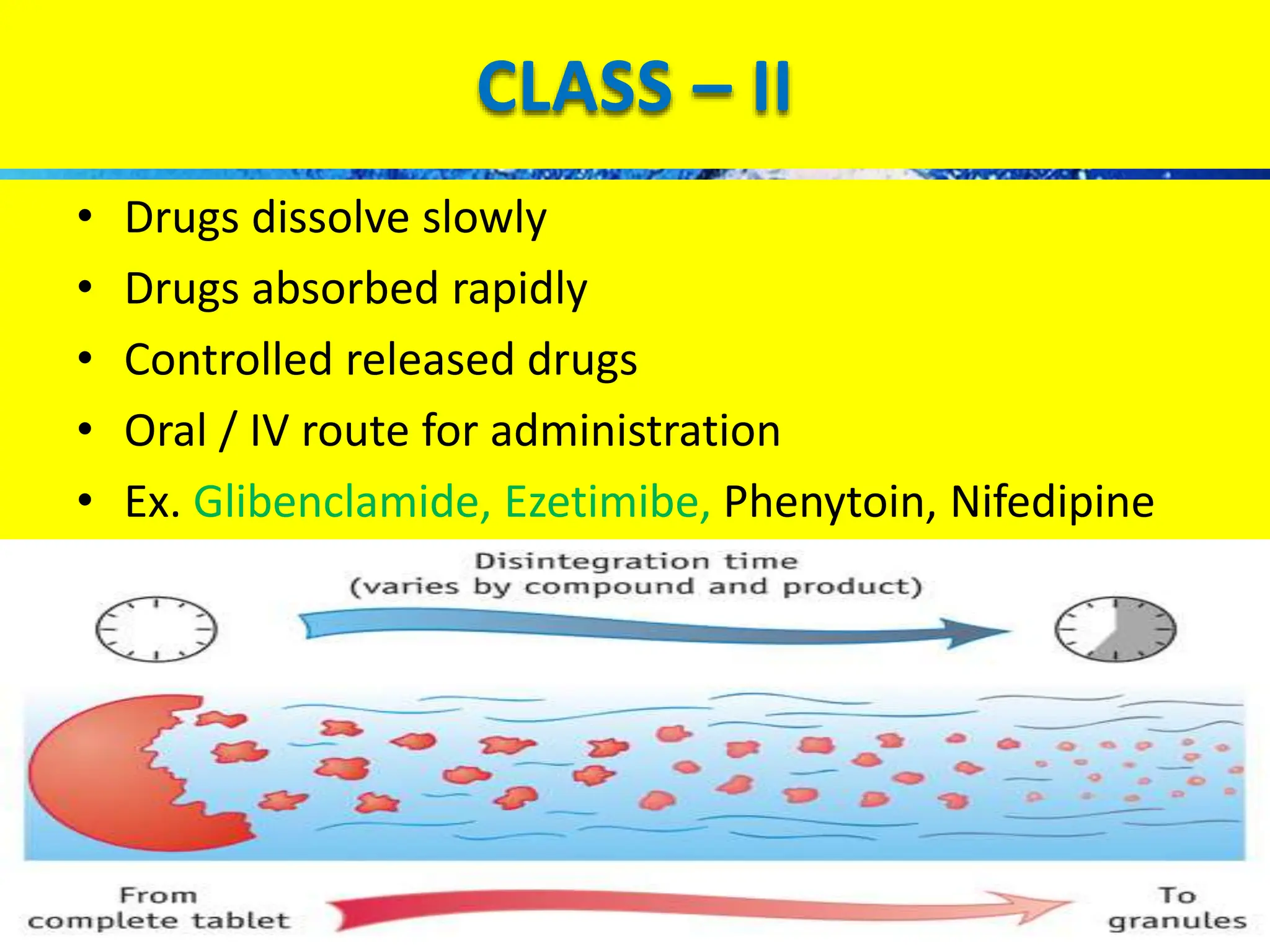
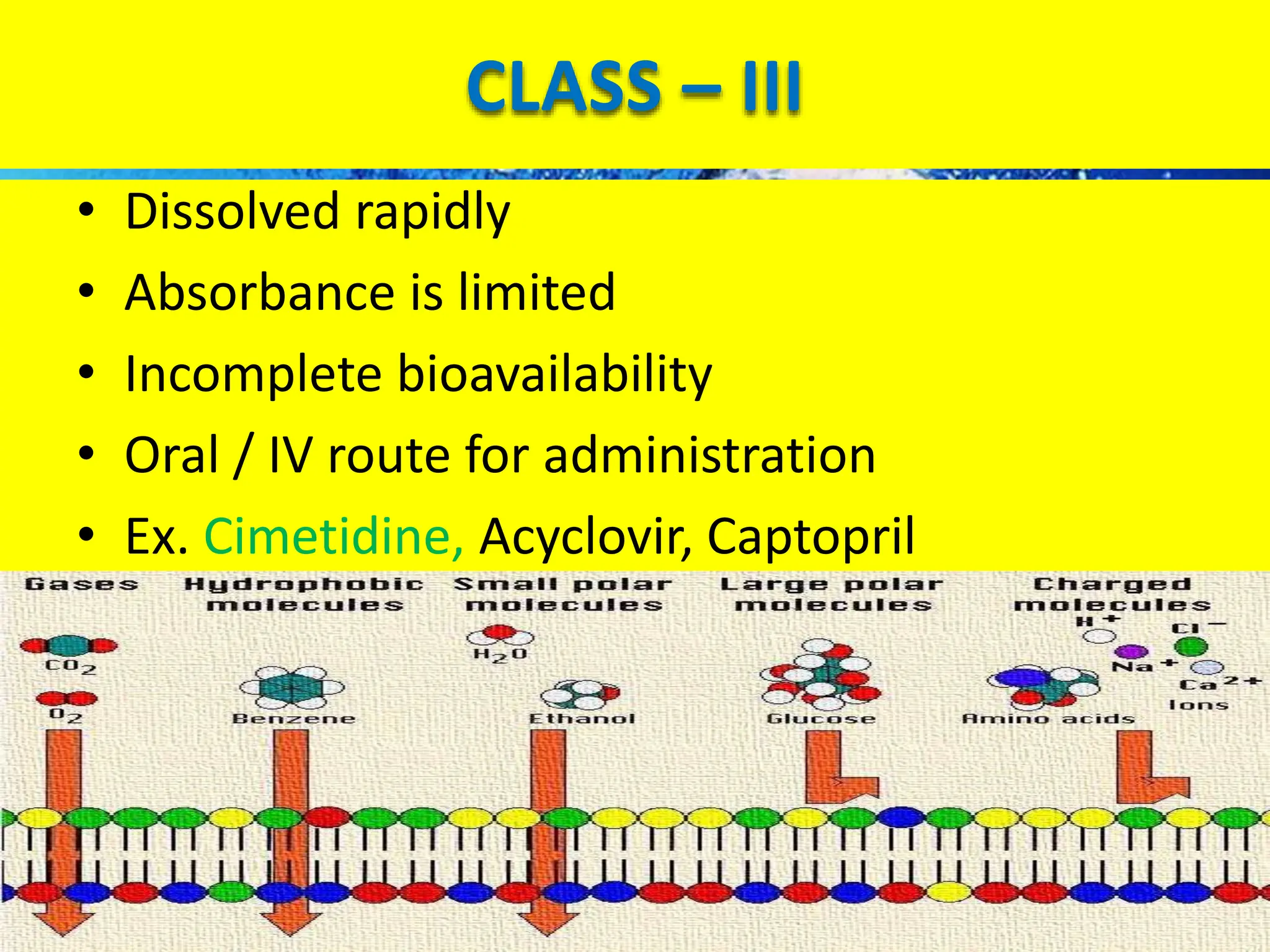
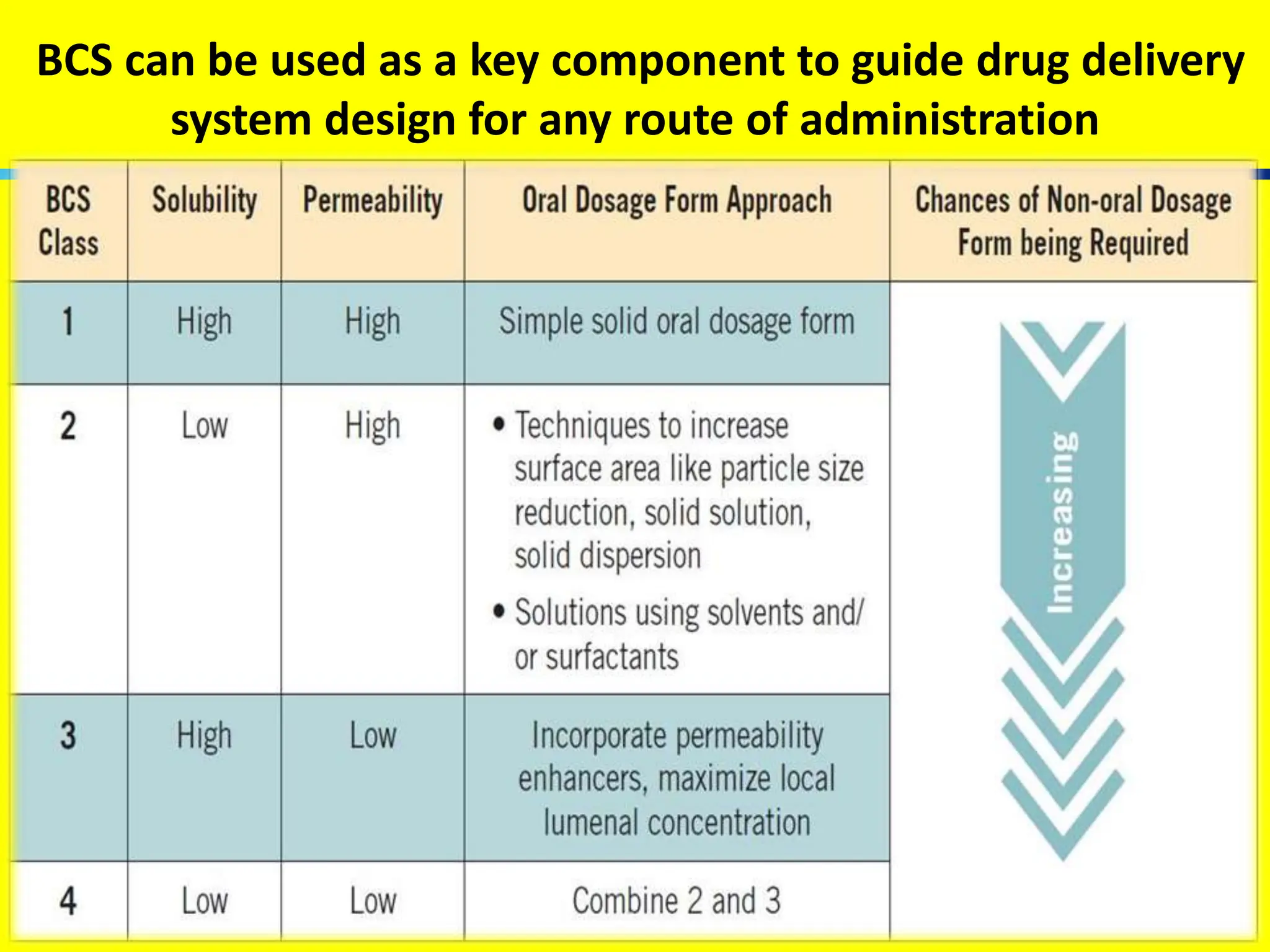
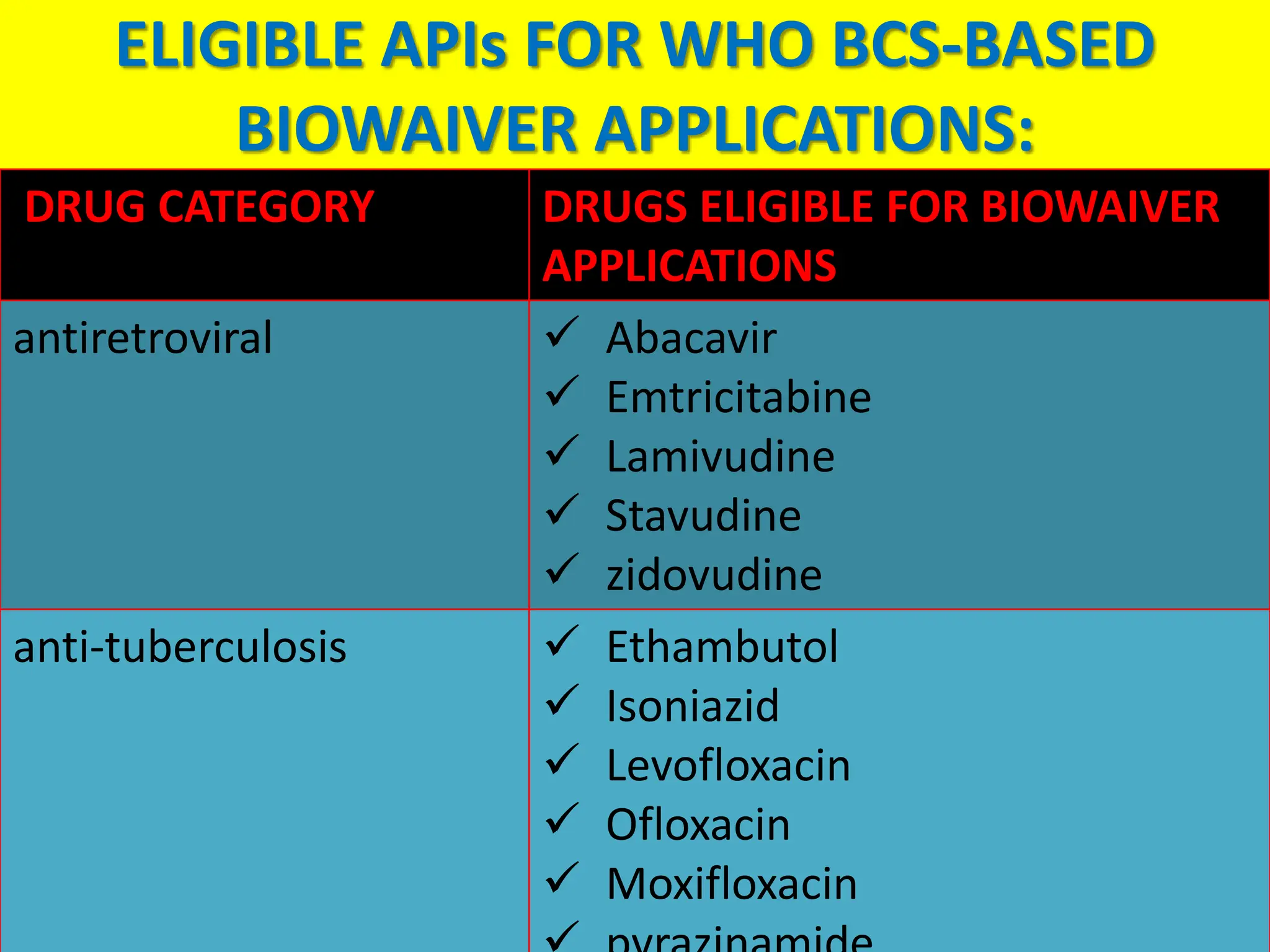
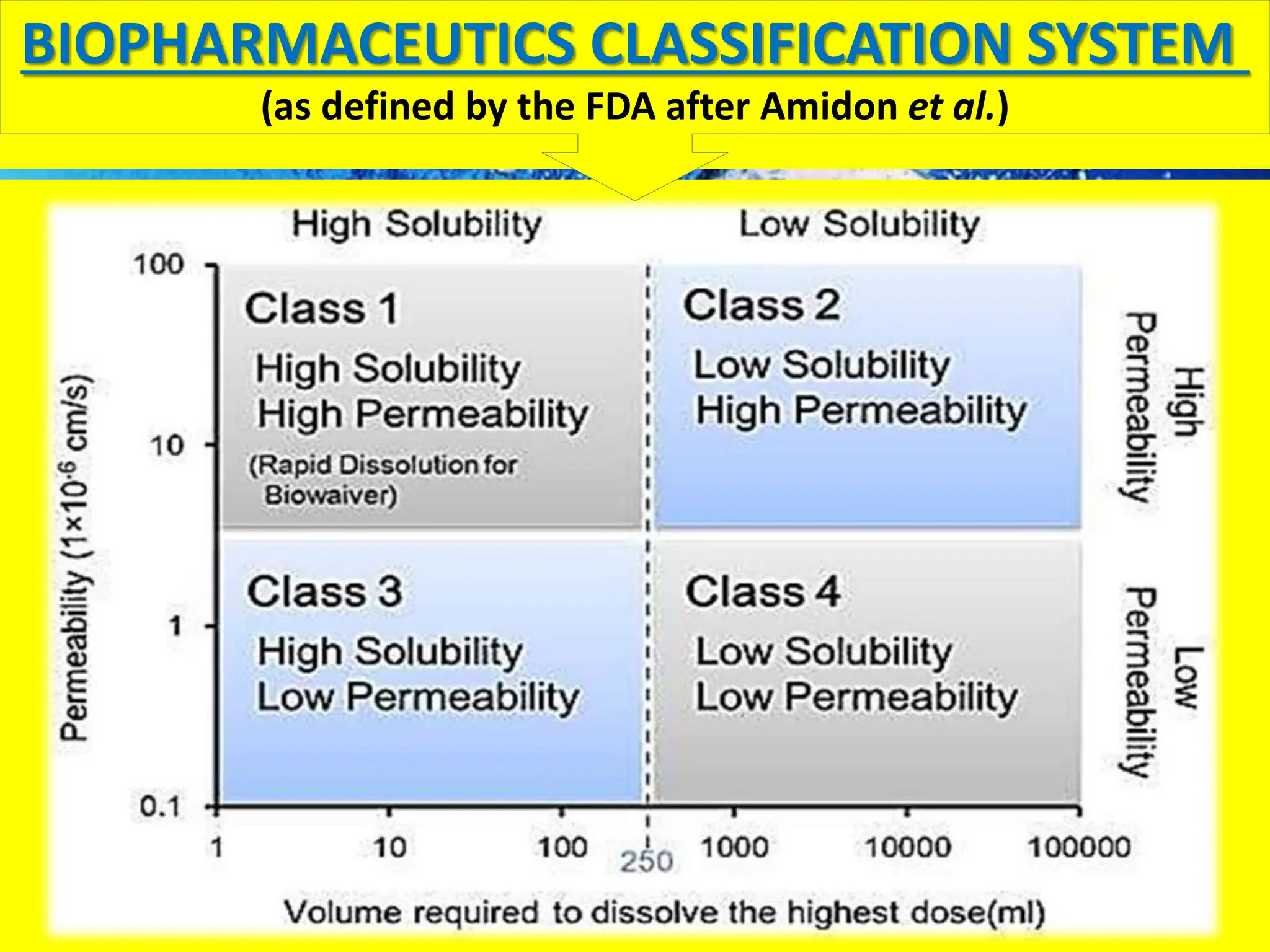
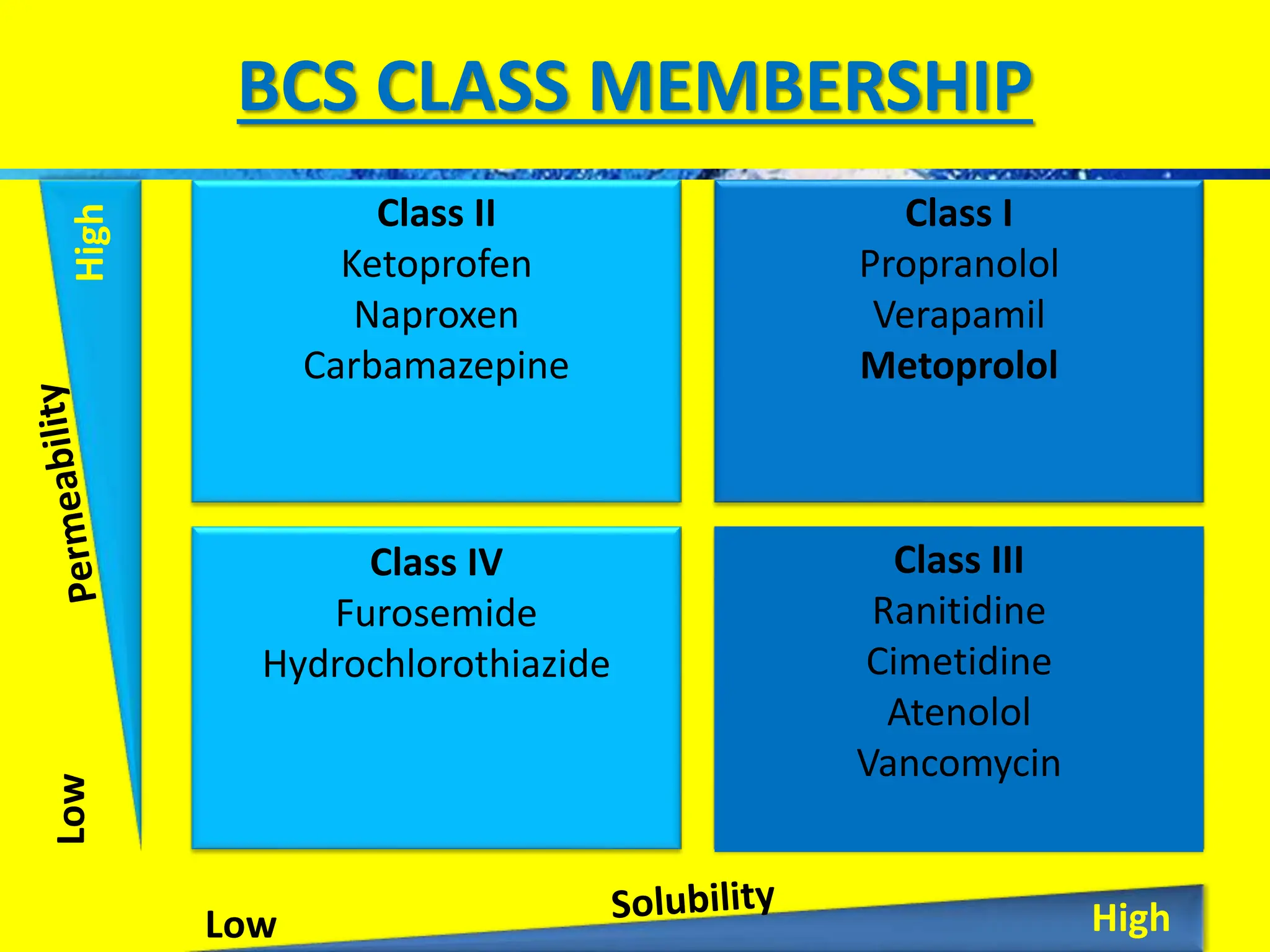
The document details the Biopharmaceutical Classification System (BCS), which classifies drug substances based on their solubility and permeability, thus influencing their bioavailability. It outlines the significance of BCS for drug regulation, enabling biowaivers to omit certain bioequivalence studies if specific criteria are met. Additionally, the document discusses the complexities and potential drawbacks in the implementation of BCS biowaivers in the pharmaceutical industry.





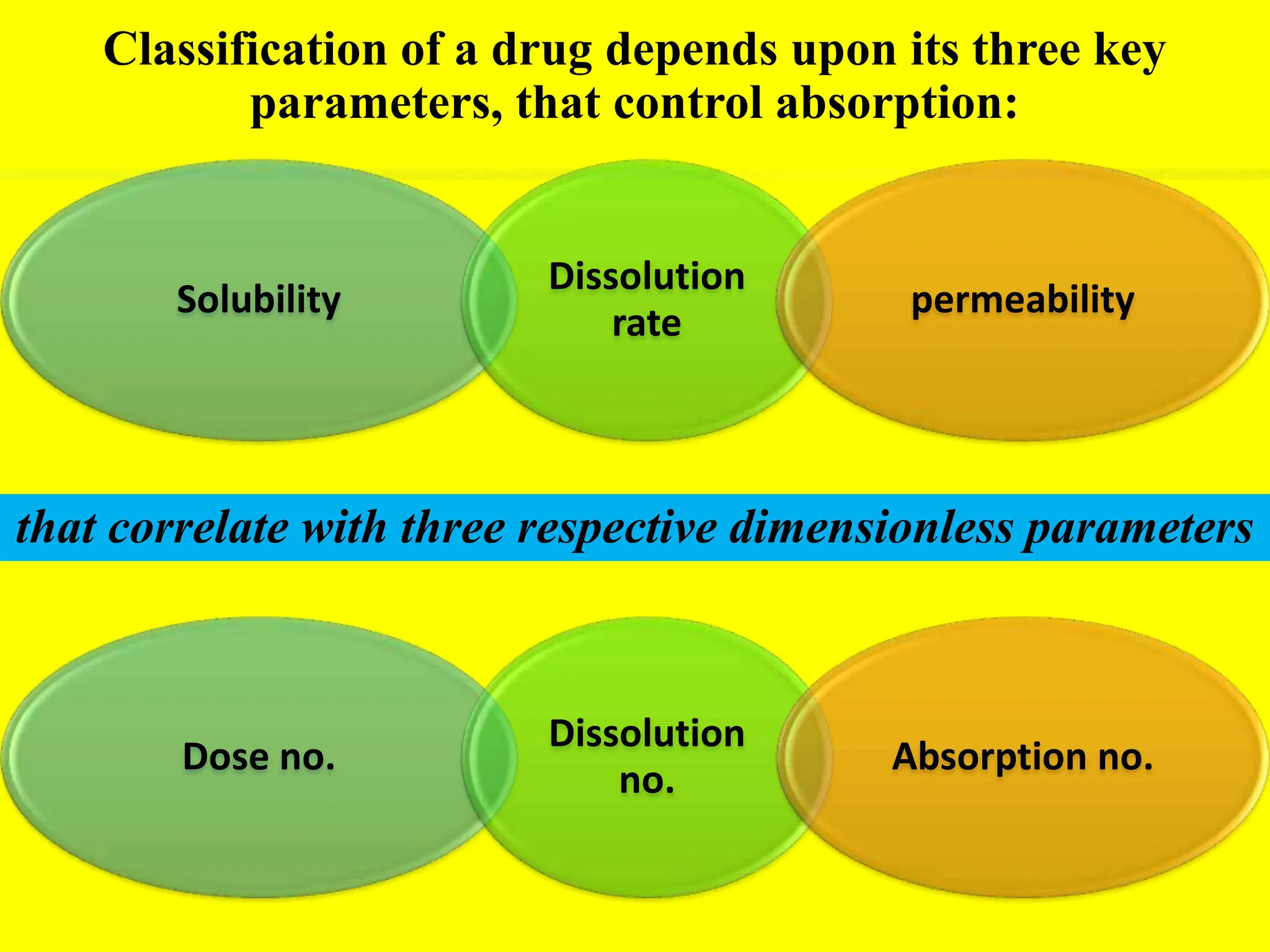
![DISSOLUTION NUMBER
A function of drug release from formulation
Should exceed 1
• Defined as the ratio of mean residence time to mean
dissolution time
Dn= [TGI/TCD]
TGI = Residence time in GI (approx. 180 min)
TCD= Time required for complete dissolution](https://image.slidesharecdn.com/biopharmaceuticalclassificationbcs-240724090843-f5c3f635/75/Bio-Pharmaceutical-Classification-BCS-pptx-10-2048.jpg)