This document discusses updates to guidelines from GOLD and GINA. Some key points discussed include:
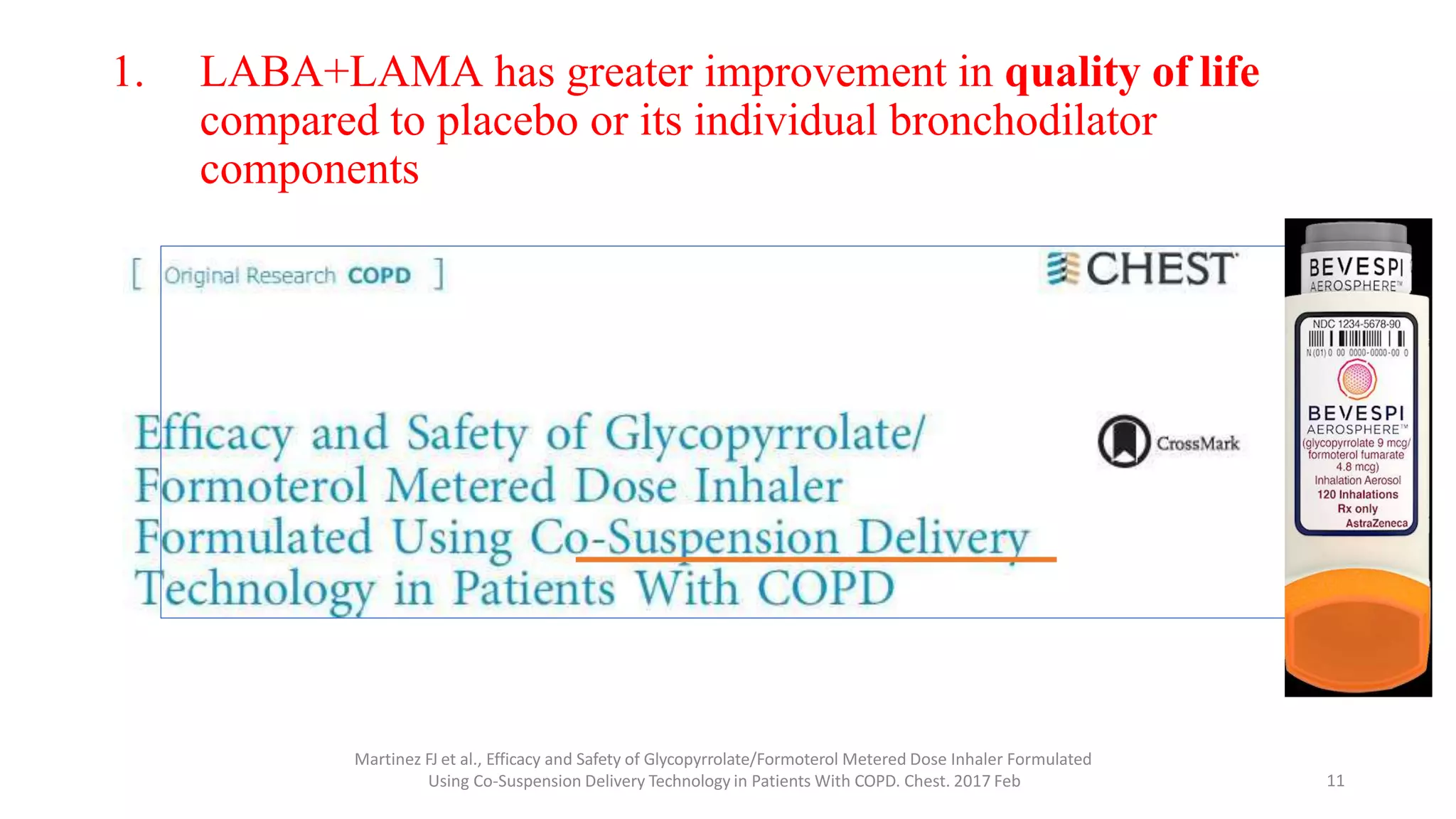
1. The GOLD guidelines now recommend LAMA/LABA combination therapy as first-line treatment for COPD based on studies showing greater improvement in quality of life compared to individual bronchodilators or placebo.
2. Studies showed the benefits of a single-inhaler triple therapy compared to ICS/LABA therapy for advanced COPD, with reductions in exacerbations.
3. The GINA guidelines now recommend considering ICS for mild asthma to reduce exacerbations based on new evidence. Anti-IL5 monoclonal antibodies like mepolizumab and benralizumab were added as add-