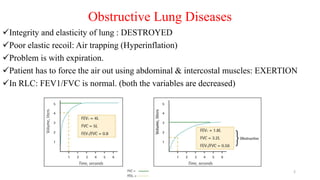
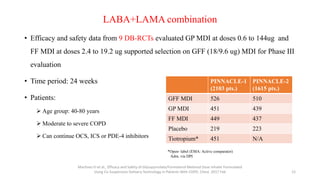
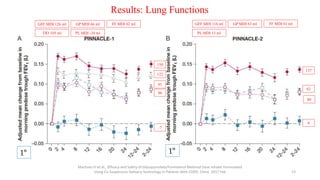
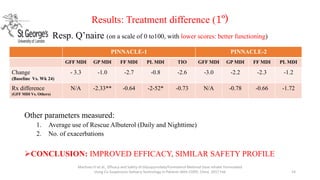
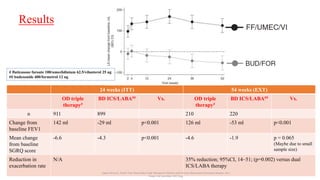
1. The document discusses updates to the 2018 GOLD and GINA guidelines for chronic obstructive pulmonary disease (COPD) and asthma management.
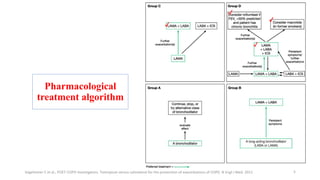
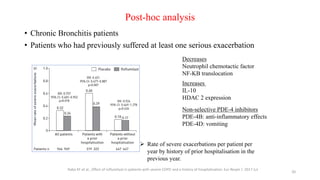
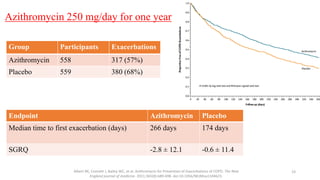
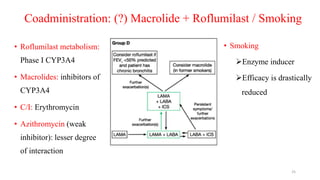
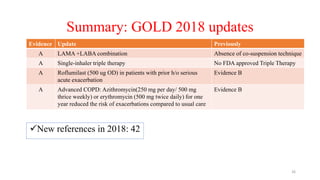
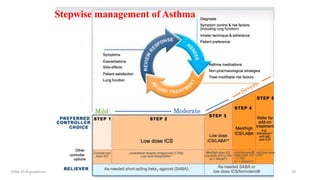
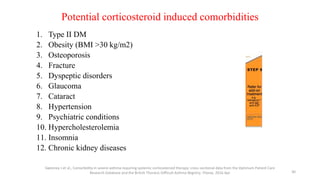
2. New recommendations include the use of single-inhaler triple therapy and macrolide antibiotics to reduce exacerbations in COPD, as well as considering inhaled corticosteroids for mild asthma.
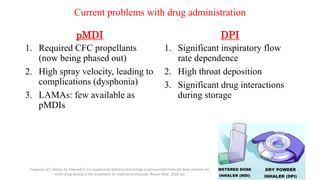
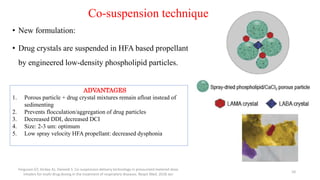
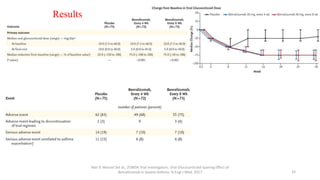
3. The benefits of new drug delivery methods, combinations therapies, and treatments targeting specific asthma and COPD phenotypes are summarized from recent clinical trials.