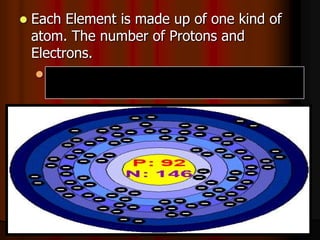
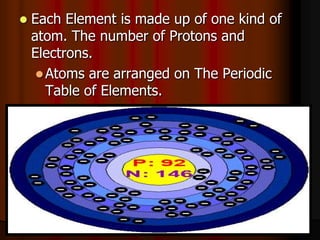
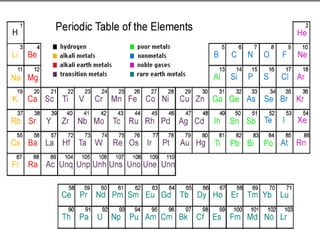
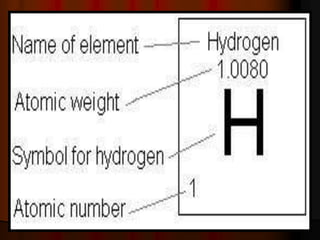
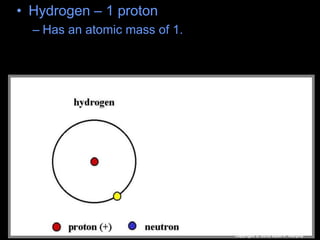
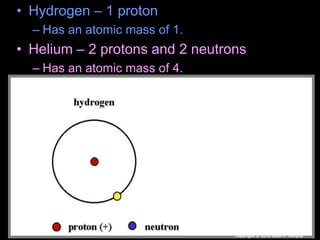
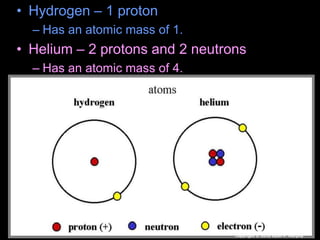
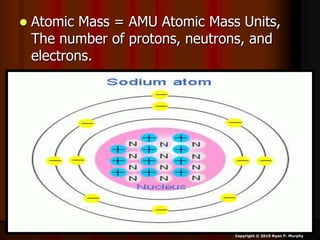
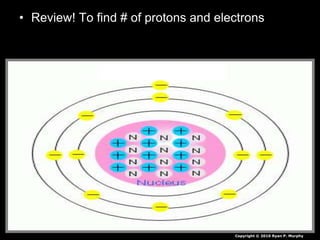
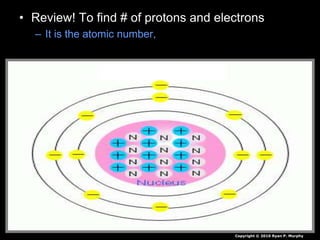
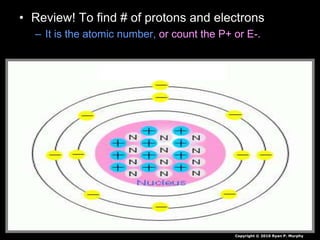
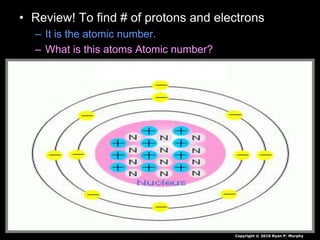
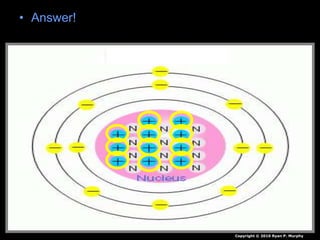
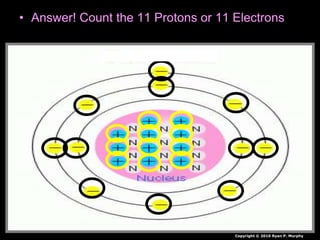
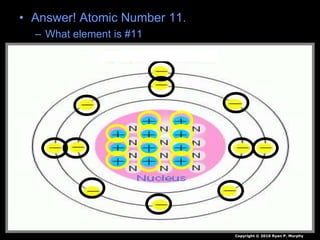
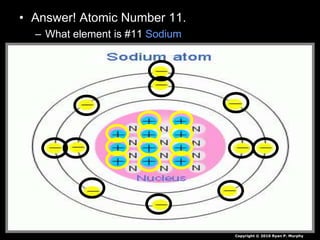
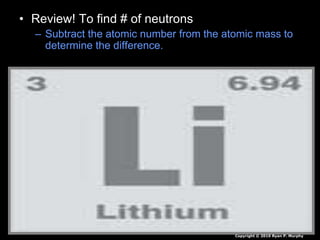
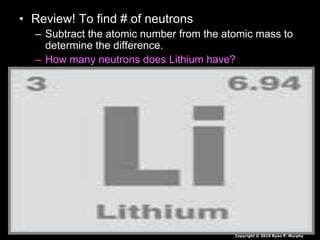
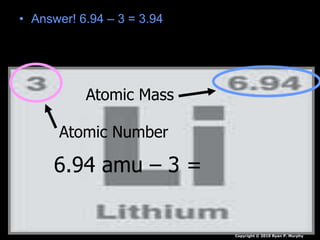
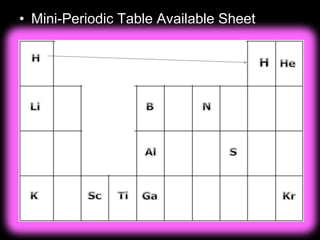
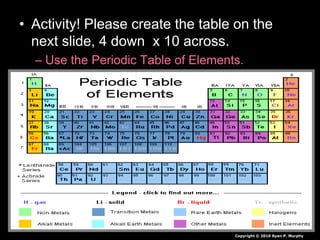
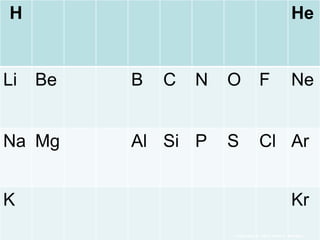
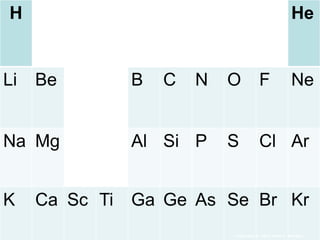
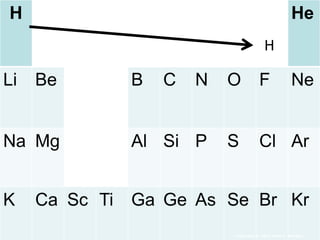
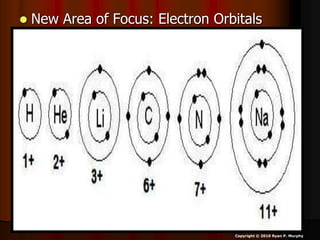
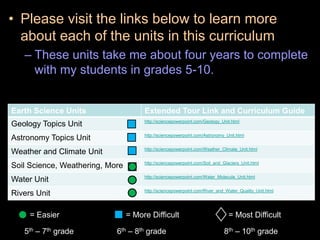
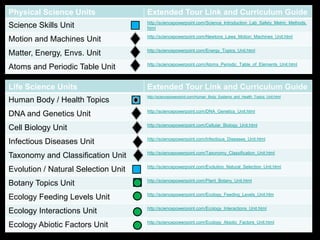
The document covers fundamental concepts of atomic theory primarily focusing on John Dalton's theories, the structure of atoms, and the periodic table. It includes notes and guidelines for students on how to record essential information clearly and engage in subsequent learning activities related to atomic structure. Additionally, references to educational resources and links for further exploration are provided.