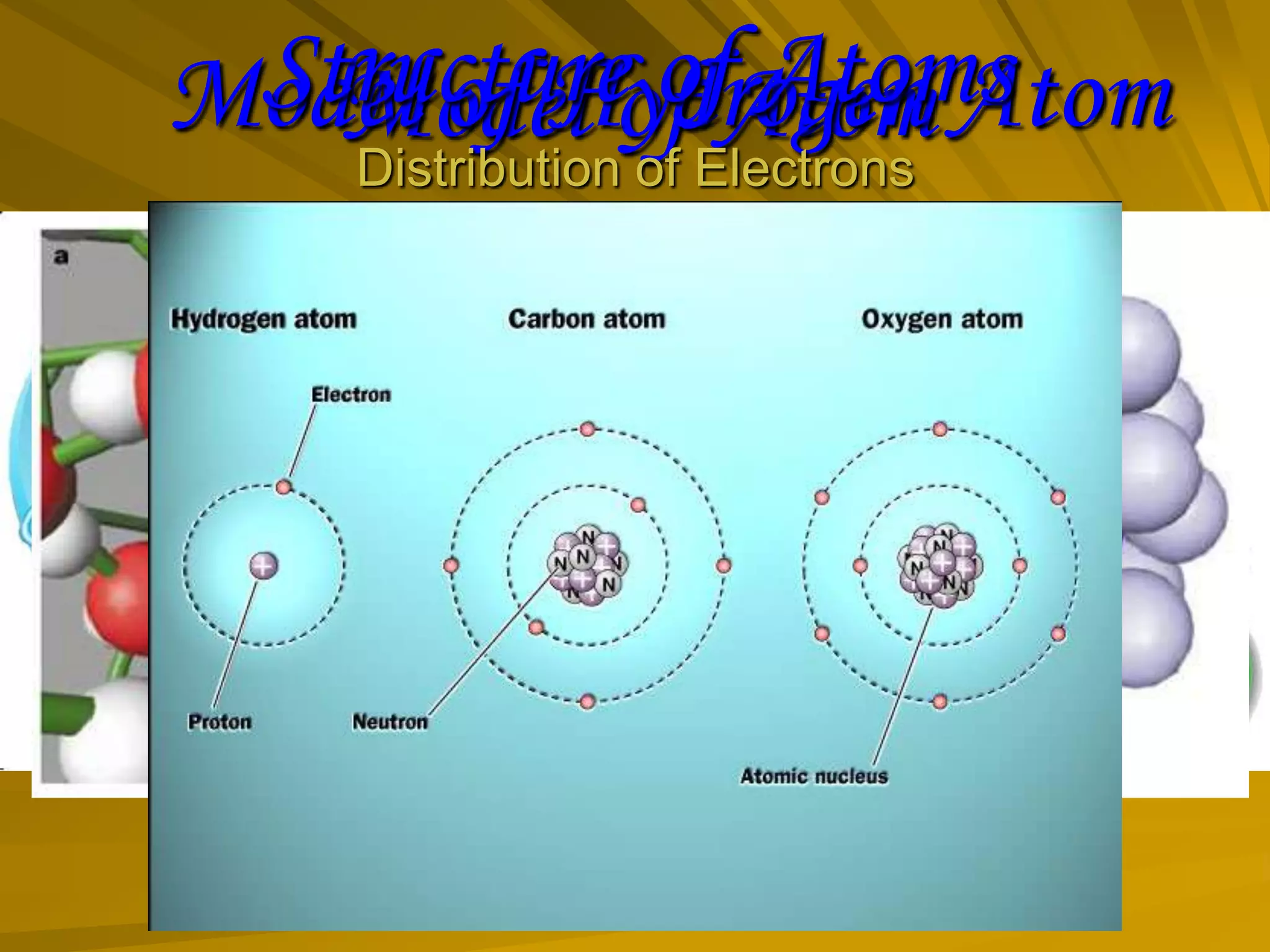
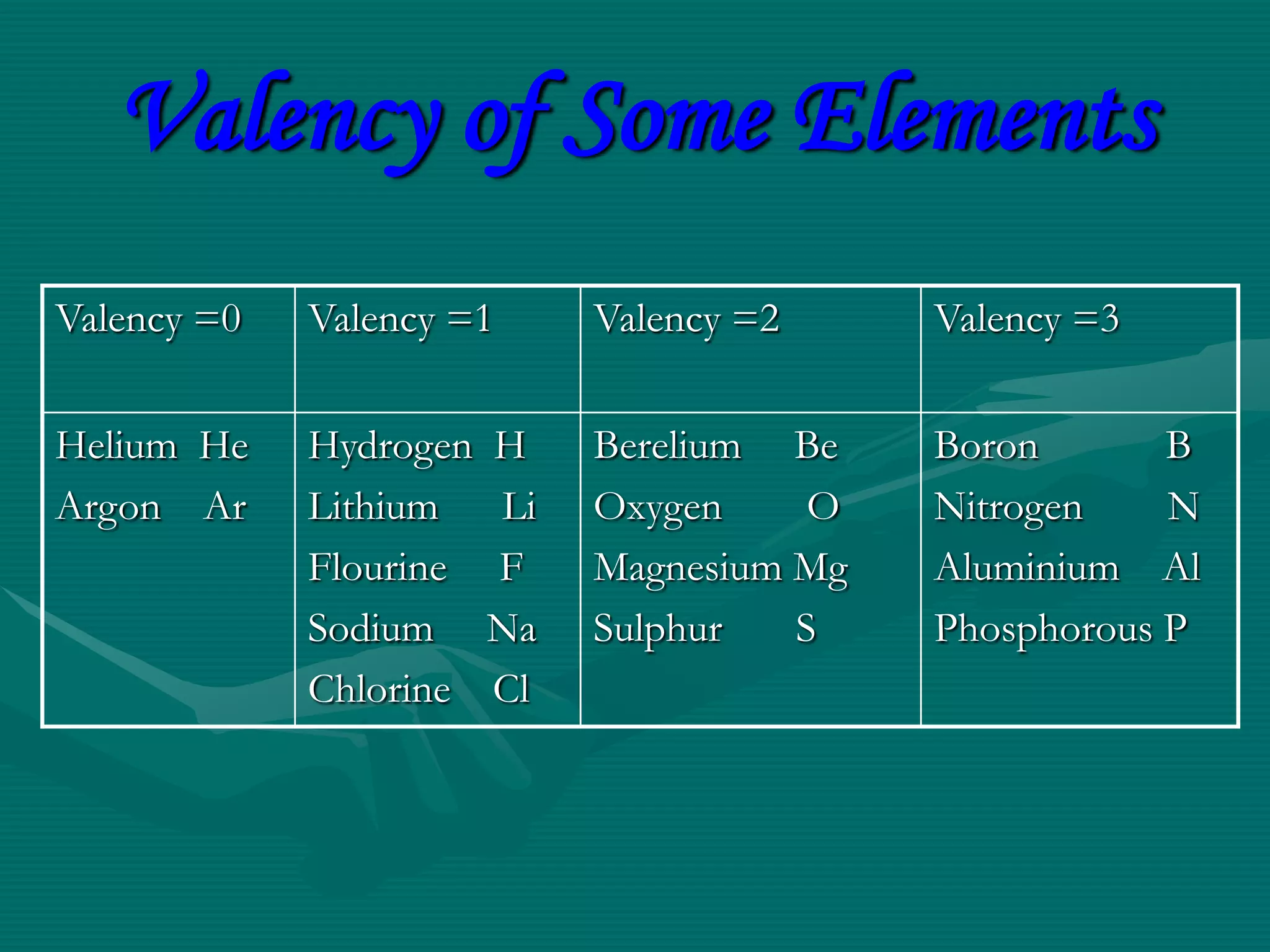
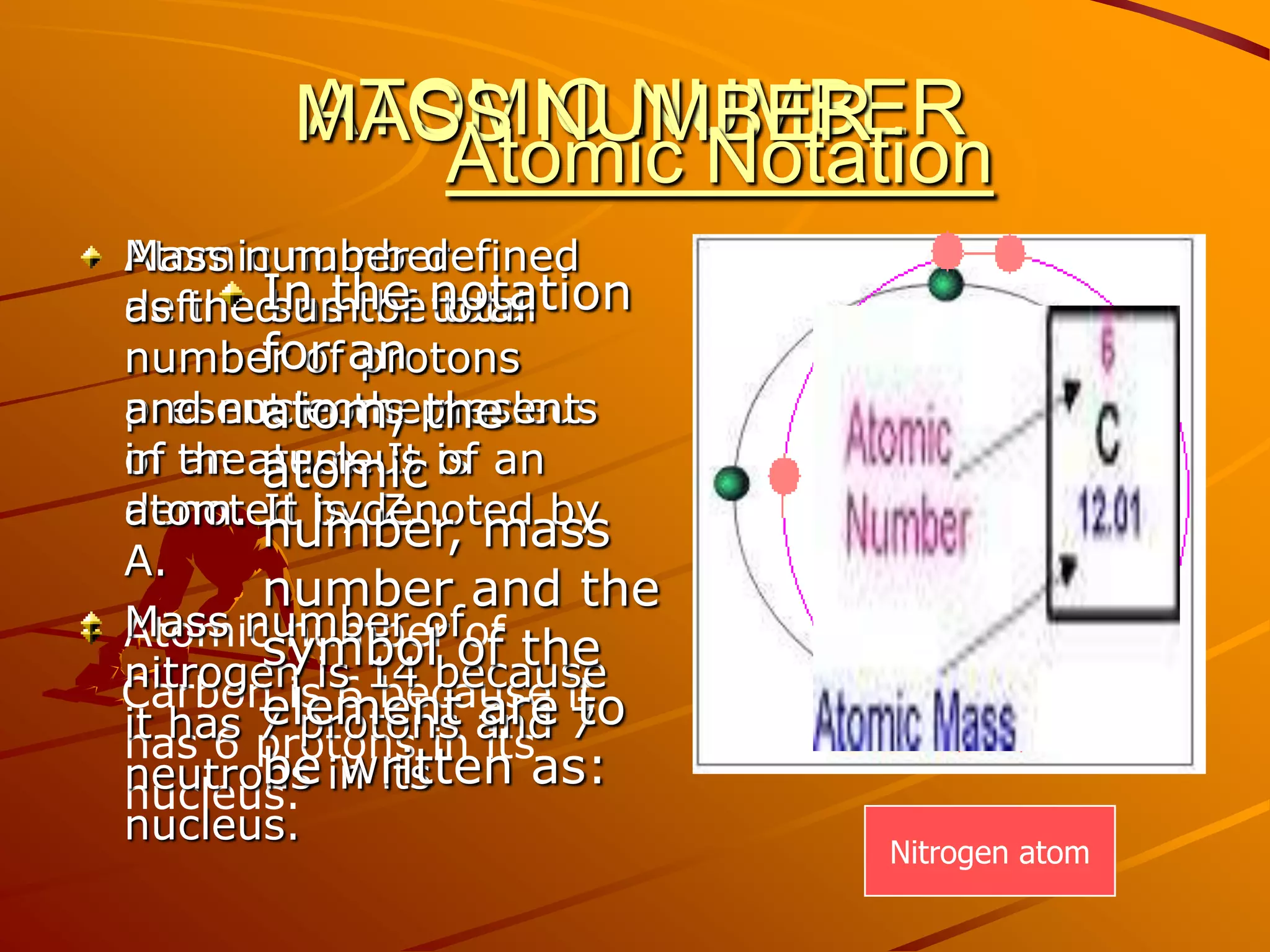
The document summarizes different models of the atom's structure, including Thomson's model of electrons embedded in a positive sphere, Rutherford's model with a small, dense nucleus at the center discovered through alpha particle scattering experiments, and Bohr's model adding allowed electron orbits to explain stability. It also discusses atomic number, mass number, and valency determining an element's combining capacity based on its outer shell electron configuration.