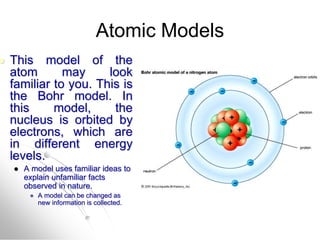
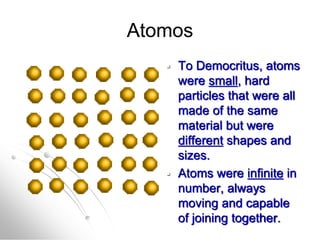
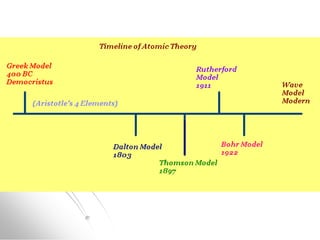
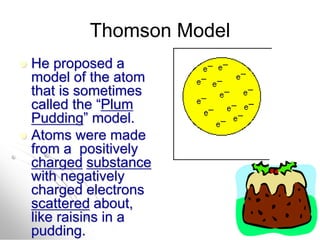
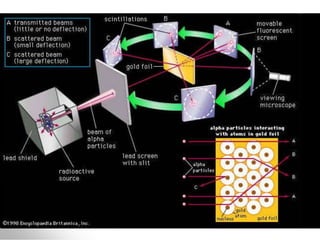
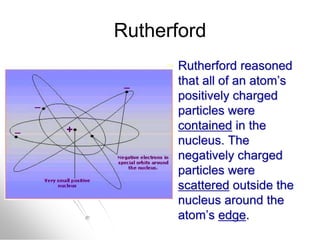
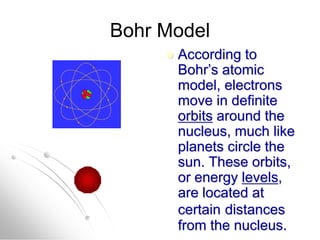
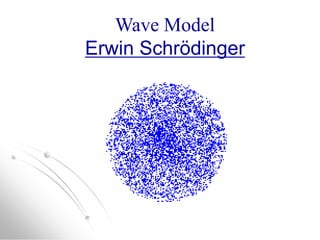
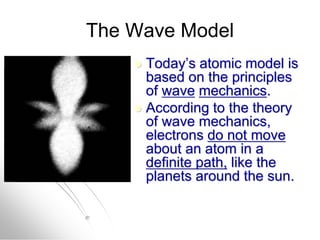
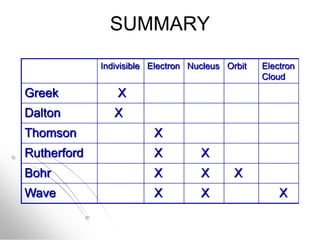
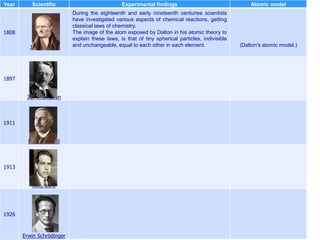
The document traces the history of atomic models from ancient Greek philosophers to modern quantum mechanics. It describes key experiments and findings that led scientists like Dalton, Thomson, Rutherford, Bohr, and Schrödinger to develop successively more accurate models of the atom. Dalton proposed atoms as indivisible particles, Thomson discovered electrons within the atom, Rutherford found the dense nucleus through gold foil experiments, Bohr incorporated electron orbits, and Schrödinger introduced the wave-like electron cloud model still used today.