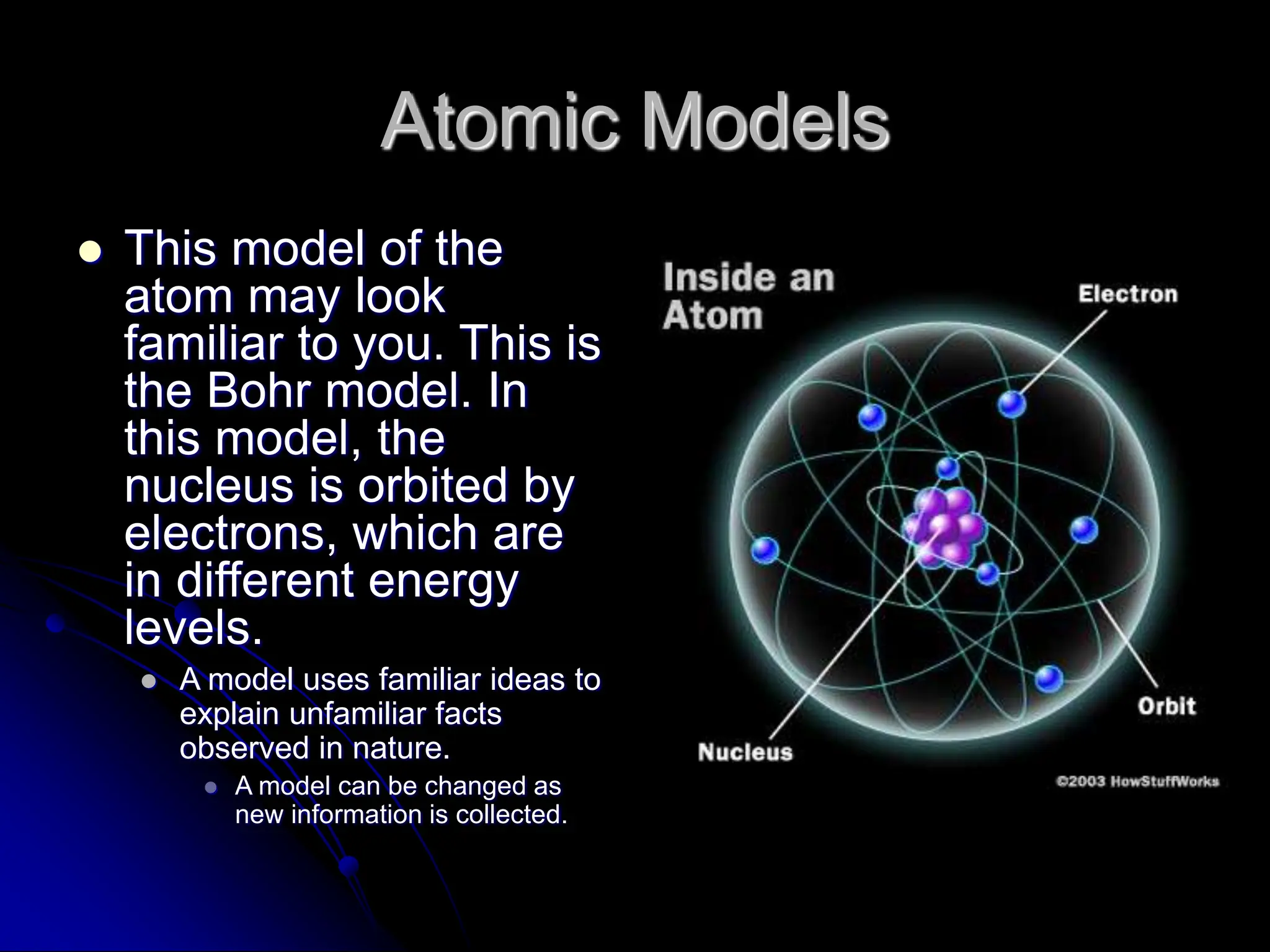
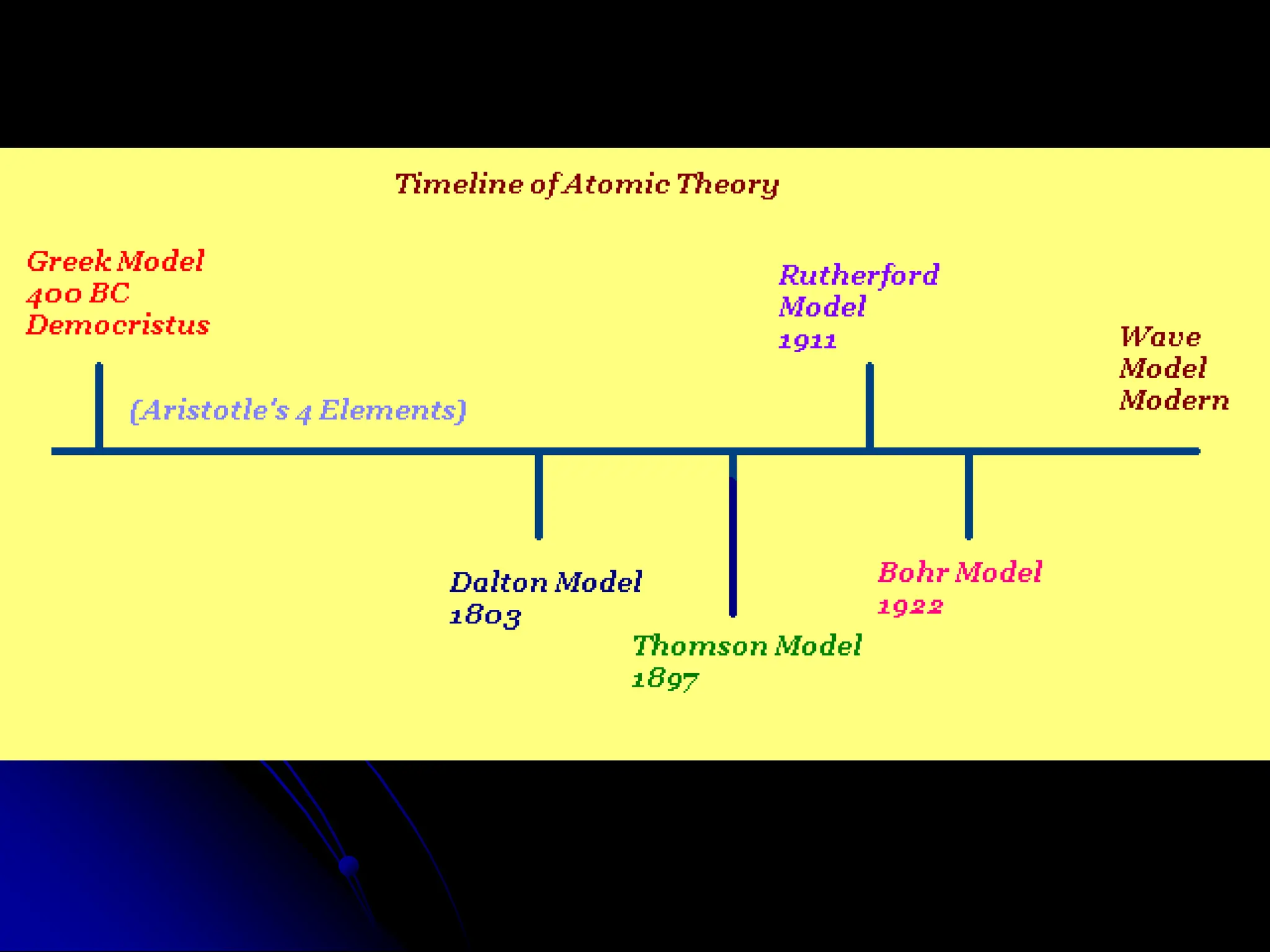
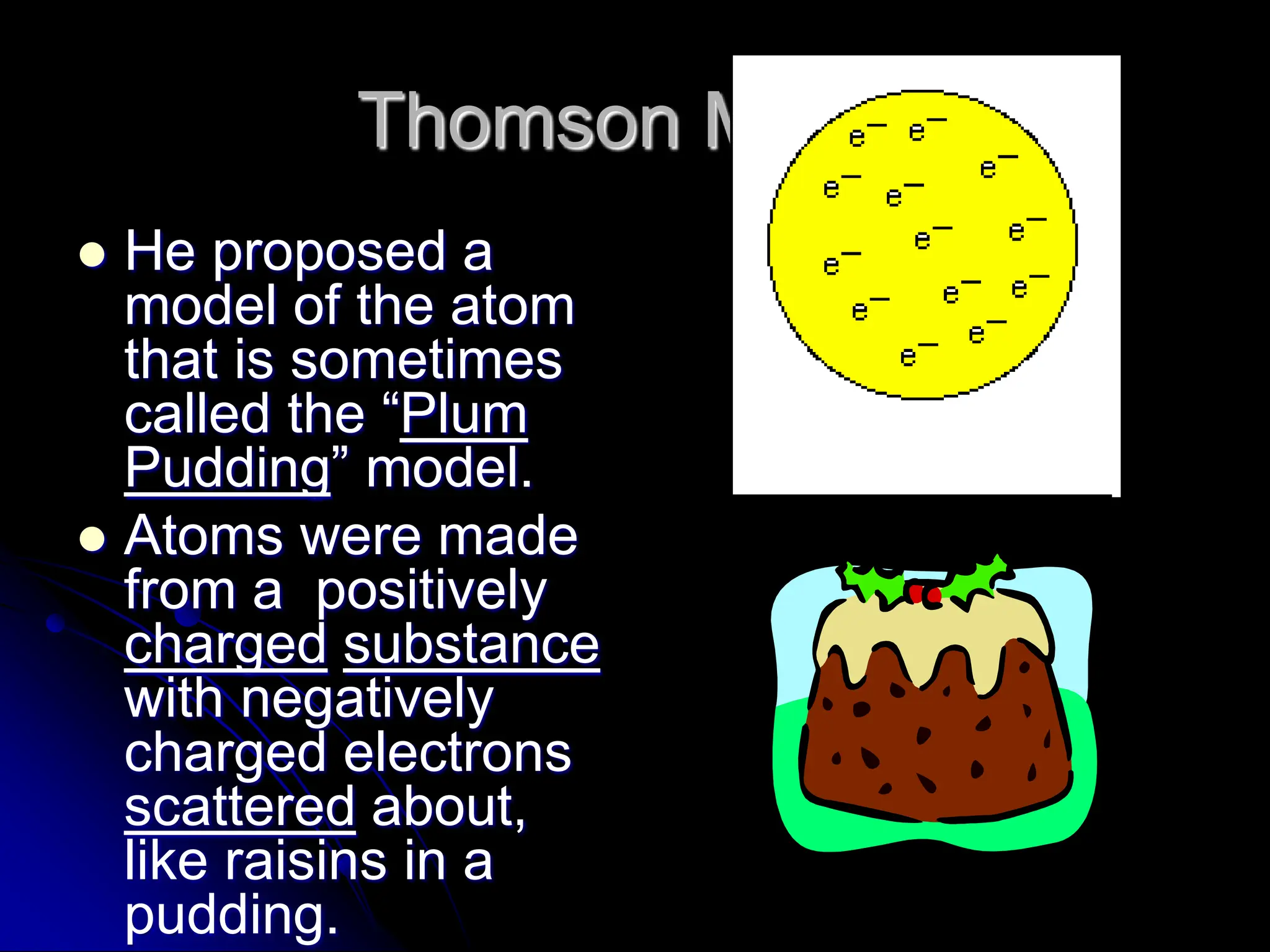
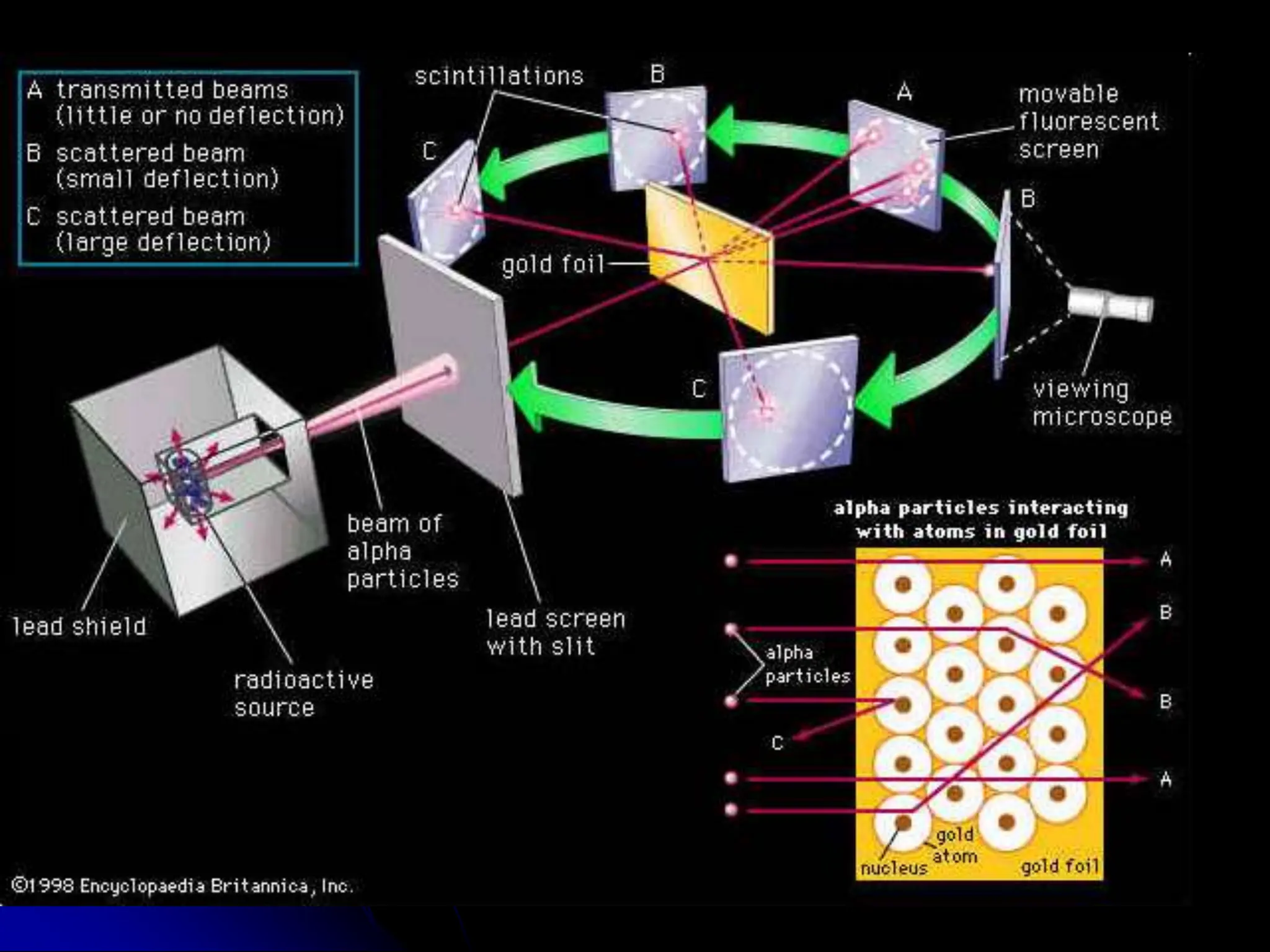
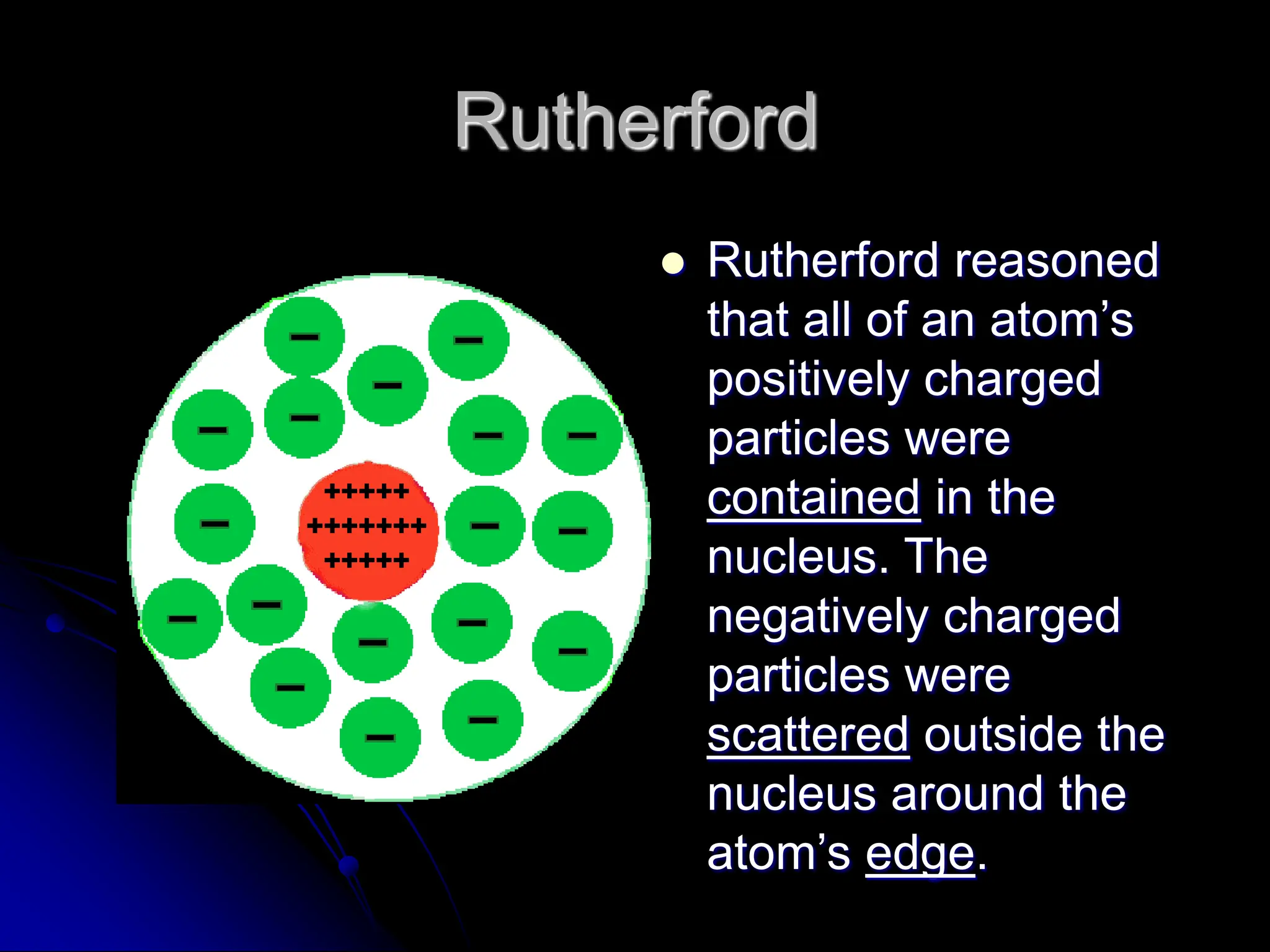
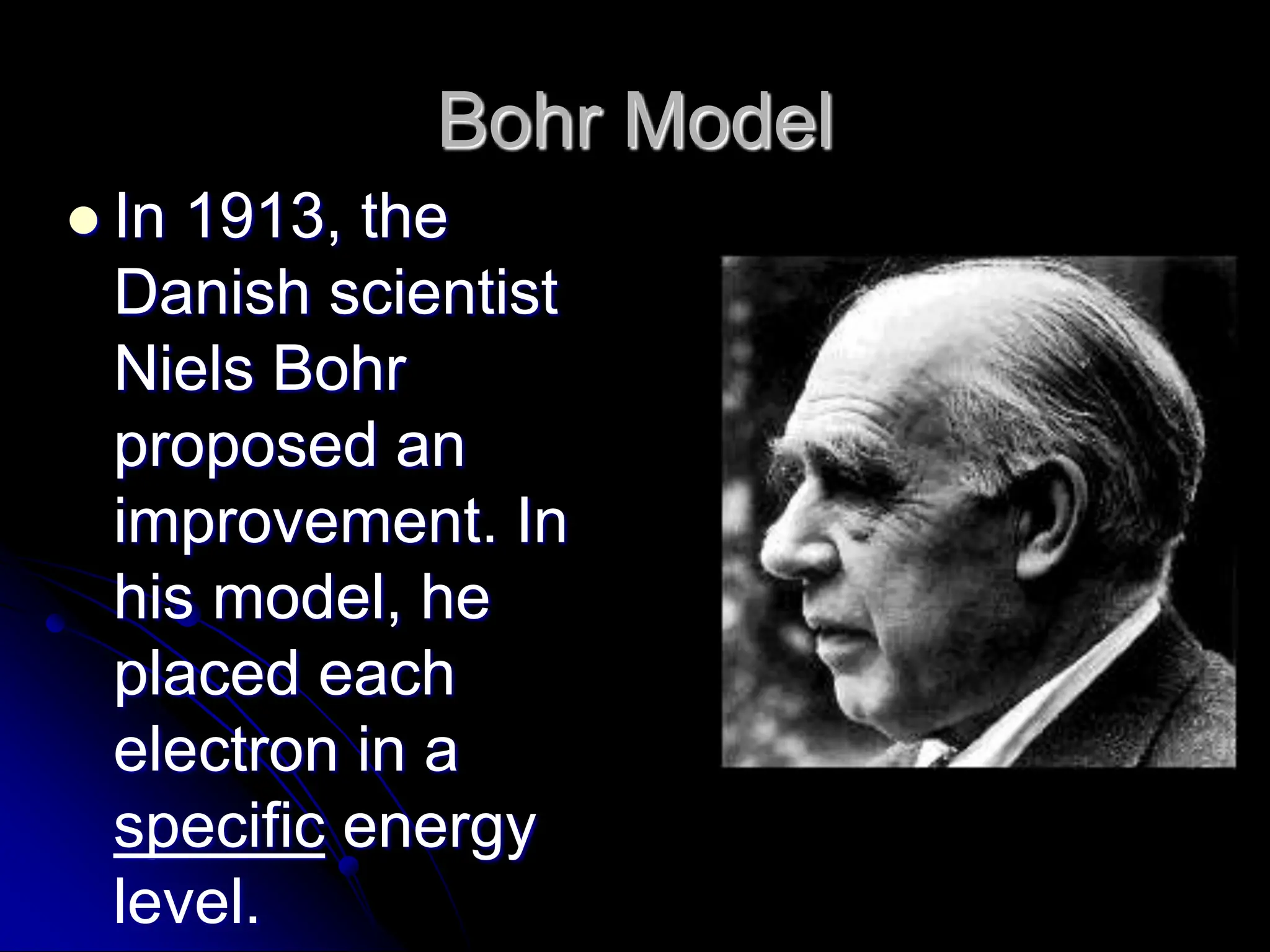
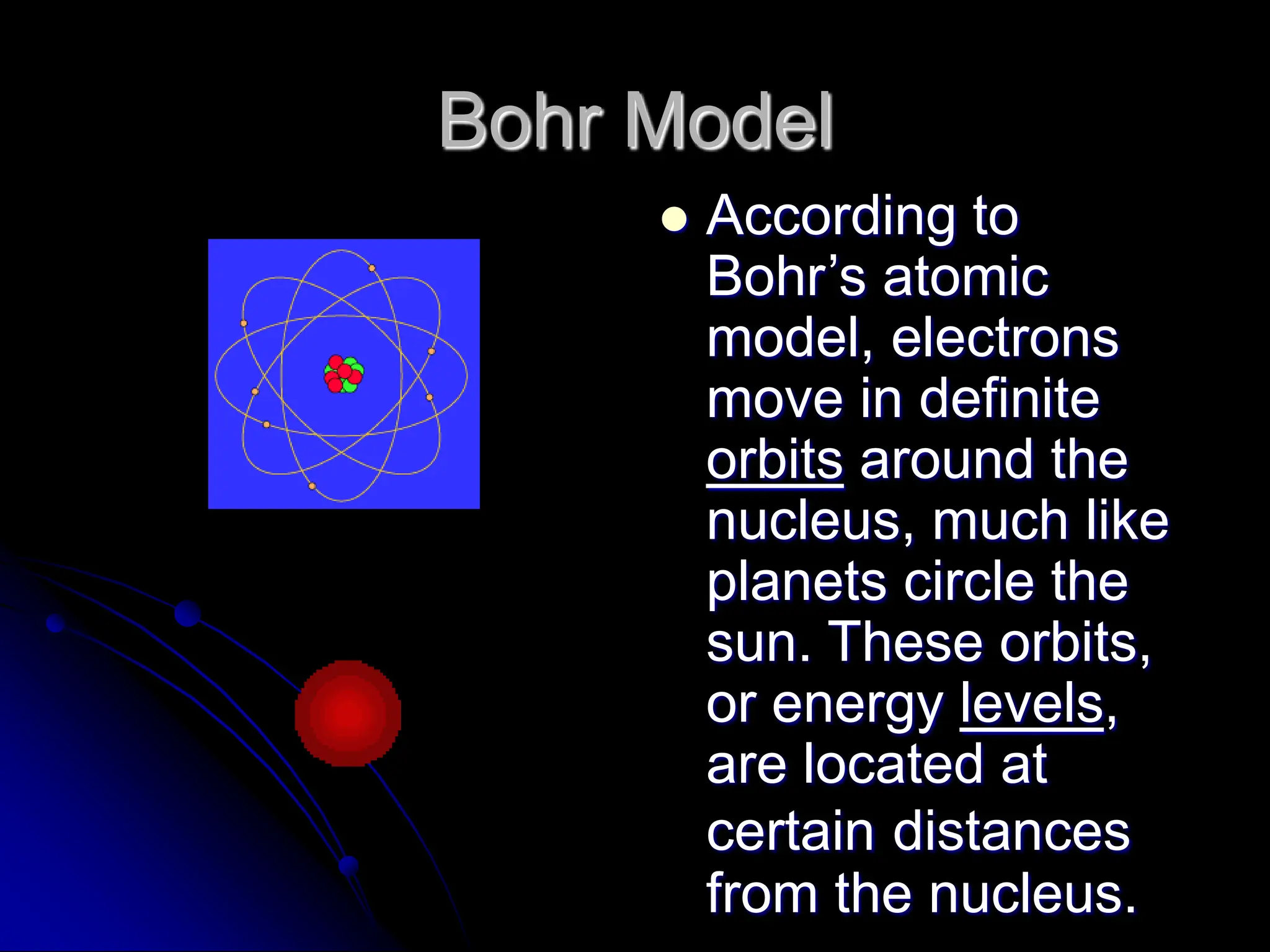
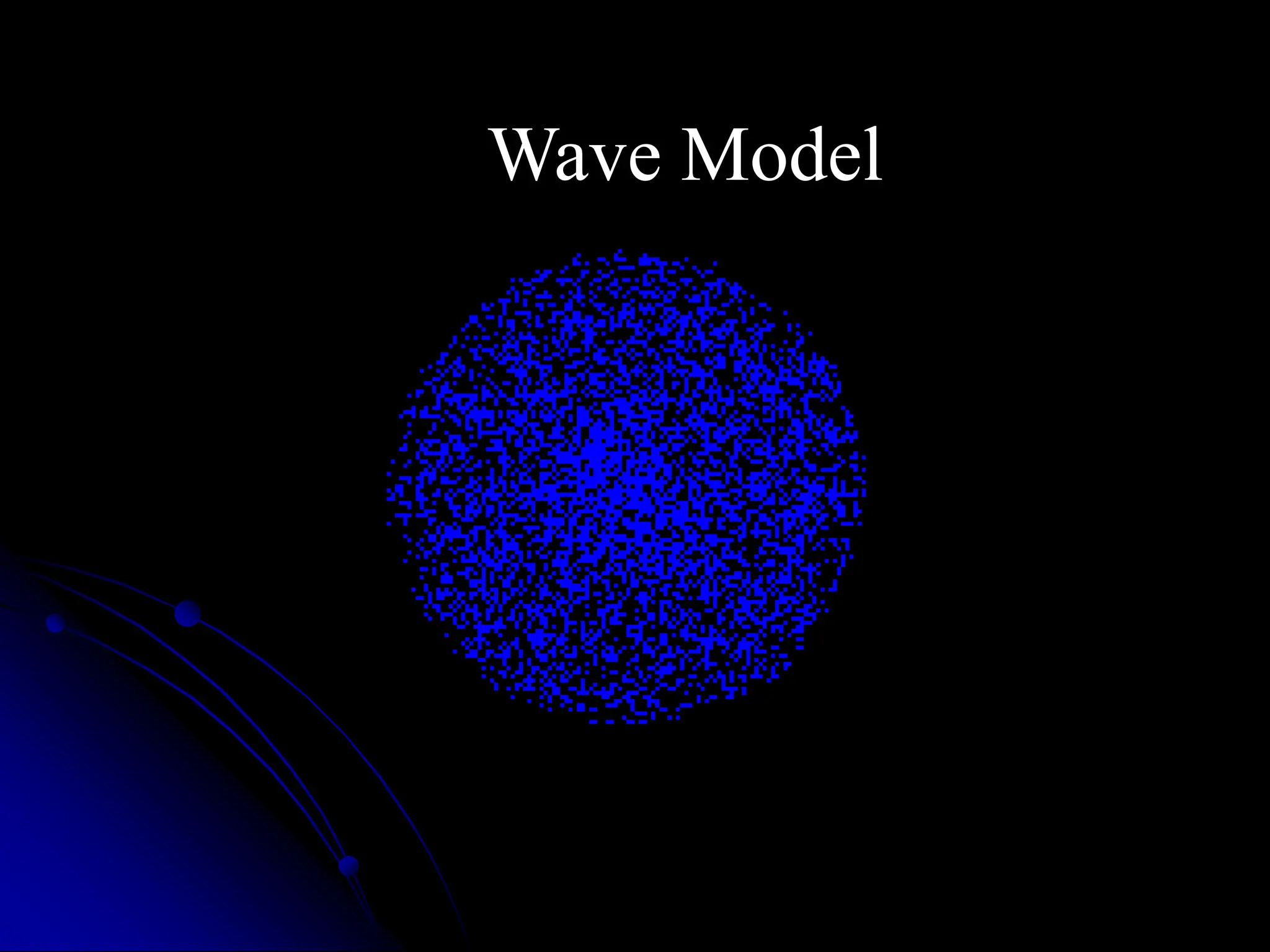
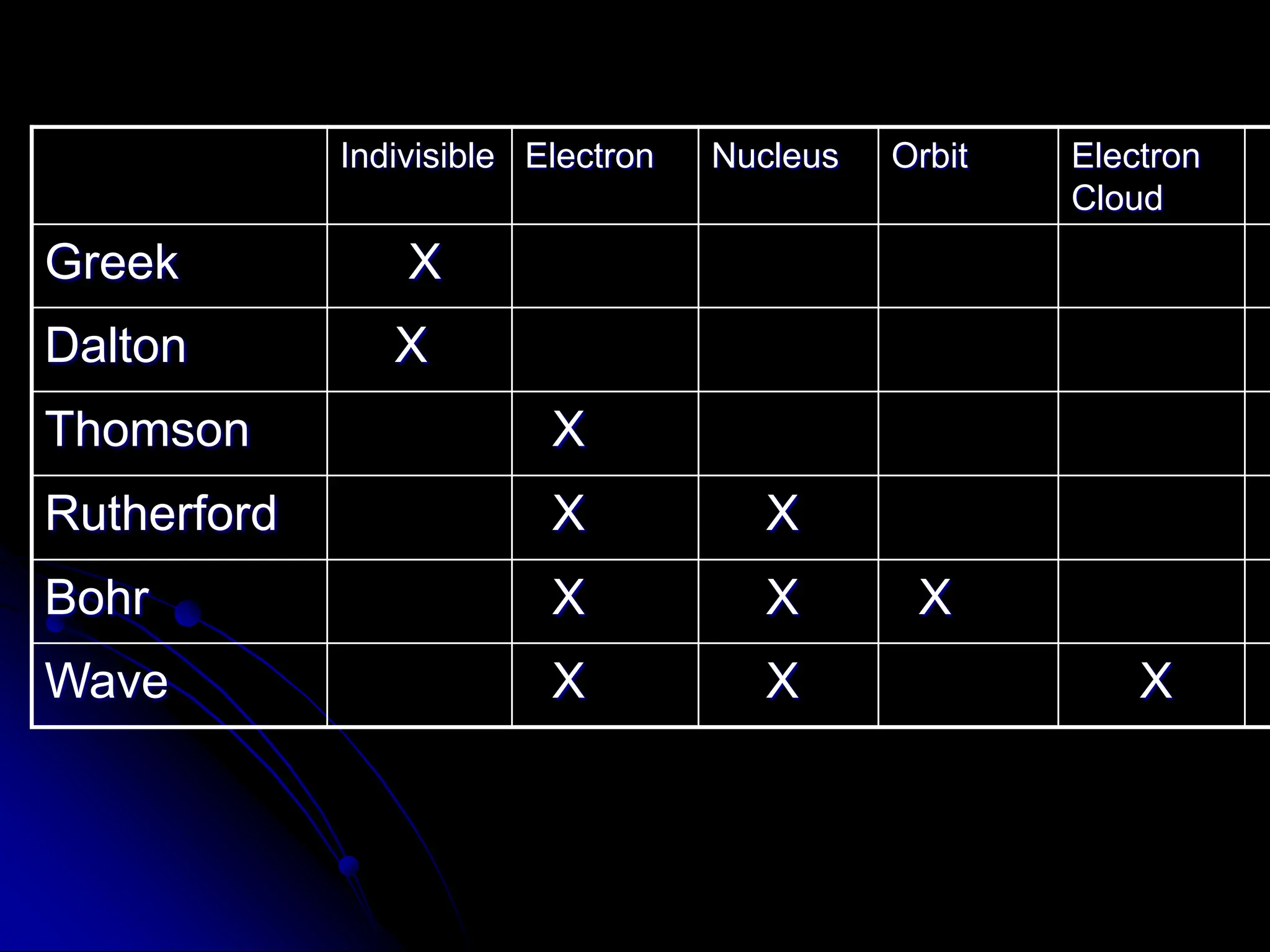
The document summarizes the development of atomic theory over time from Democritus' idea of indivisible atoms in 400 BC to the current wave model. It describes key models including Thomson's plum pudding model, Rutherford's discovery of the nucleus from his gold foil experiment, Bohr's model of electrons in distinct orbits around the nucleus, and the modern wave model where electrons exist as probabilities in electron clouds. The location of electrons depends on their energy level rather than fixed orbits.