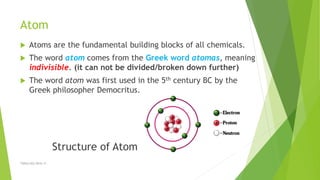
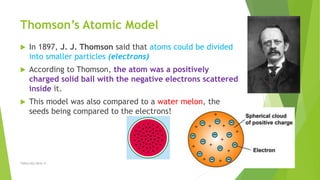
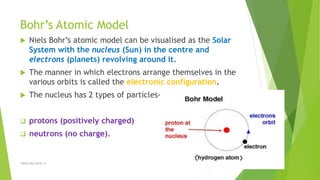
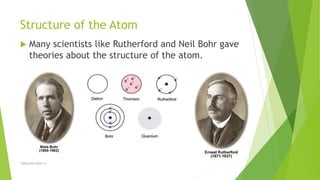
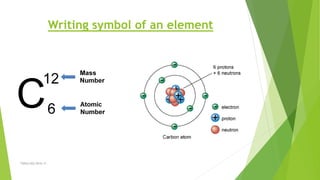
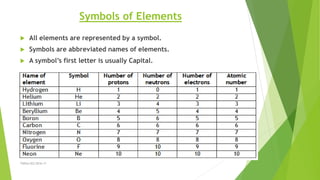
The document discusses the history and modern understanding of atomic structure. It describes early atomic theories from Dalton, Thomson, and Bohr, which proposed that atoms are the fundamental units of matter and consist of even smaller particles like electrons and a nucleus. The modern atomic model represents atoms as a tiny, positively charged nucleus surrounded by negatively charged electrons in orbitals. An atom's atomic number is the number of protons, which equals the number of electrons. The mass number includes both protons and neutrons in the nucleus. Each element is represented by a unique one or two letter atomic symbol.