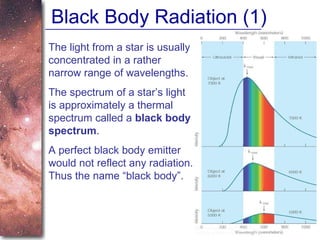
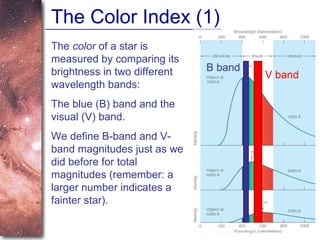
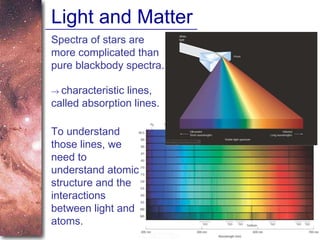
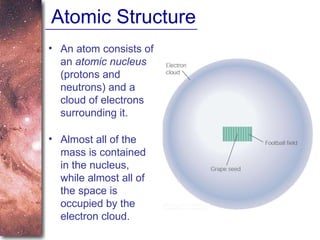
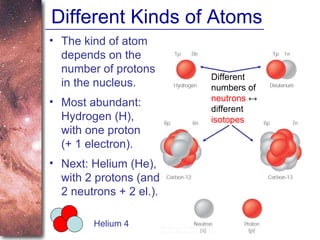
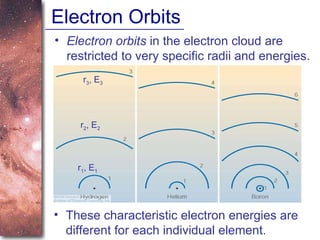
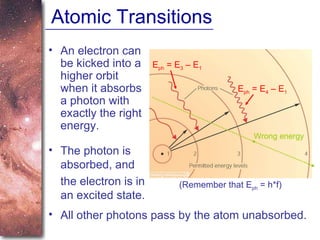
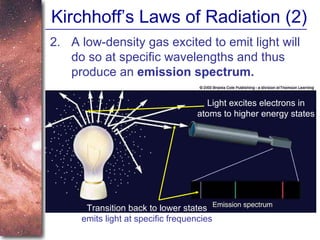
1. The document introduces concepts about how atoms interact with light and how this interaction can provide information about stars. It discusses atomic structure, electron shells, and how atoms absorb and emit light at specific wavelengths.
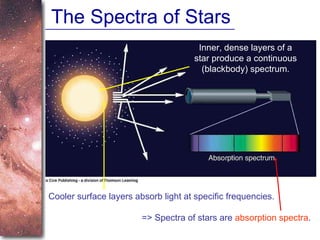
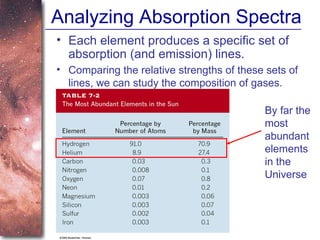
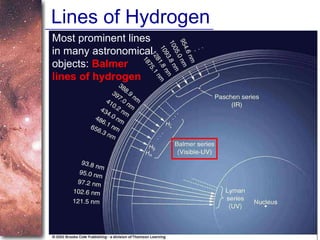
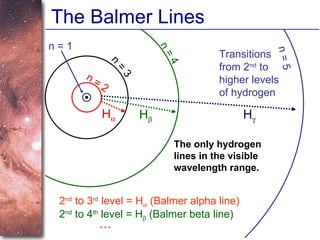
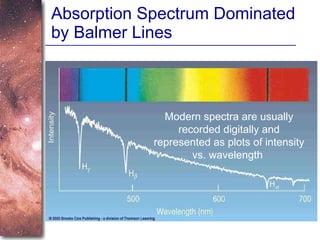
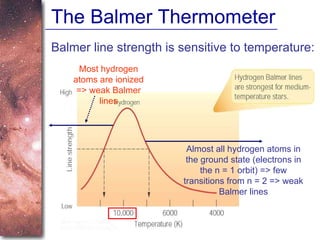
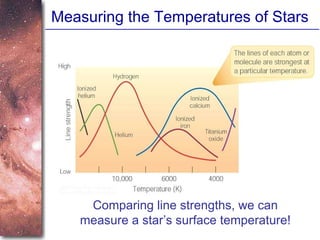
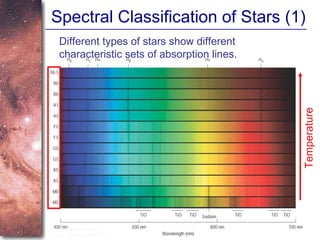
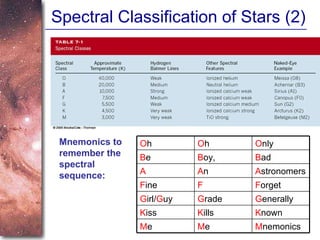
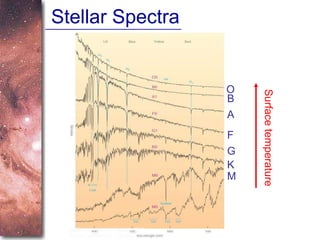
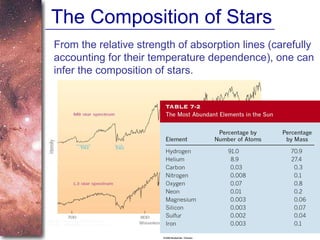
2. Spectra of stars contain absorption lines produced by atoms in their atmospheres. By analyzing these lines, astronomers can determine properties of stars like temperature, chemical composition, and velocity.
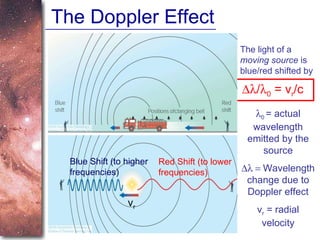
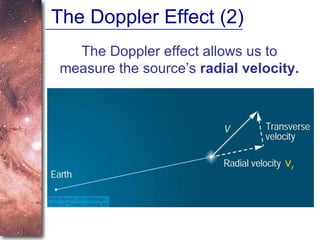
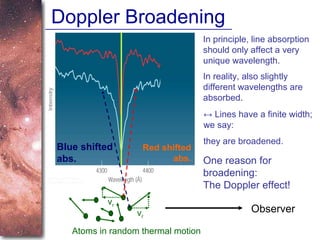
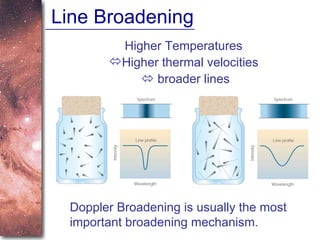
3. The Doppler effect causes shifts in absorption line wavelengths that reveal if a star is moving towards or away from Earth, allowing calculation of its radial velocity.