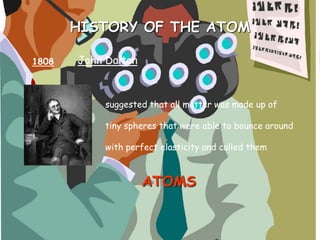
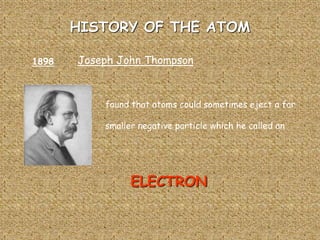
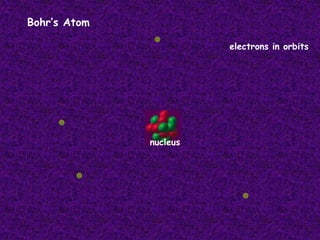
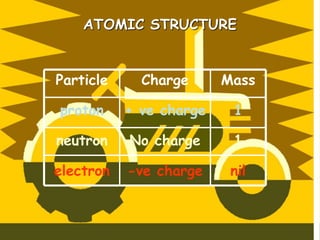
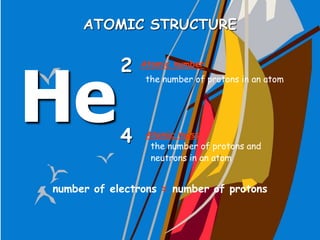
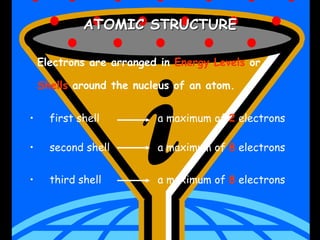
The document traces the history of atomic theory from Democritus' idea of indivisible atoms in 460 BC to Bohr's model of electrons orbiting the nucleus in 1913. Key developments included Dalton suggesting atoms were tiny spheres in 1808, Thompson discovering the electron in 1898 and proposing the plum pudding model, and Rutherford's gold foil experiment in 1910 leading him to propose that atoms have a small, dense nucleus.