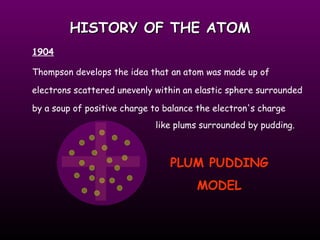
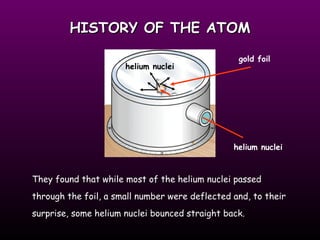
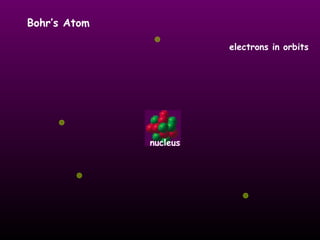
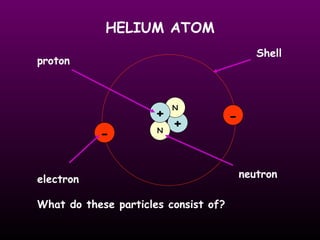
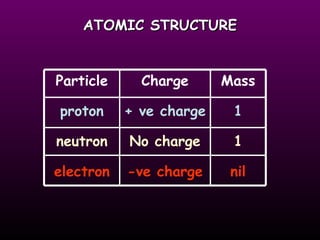
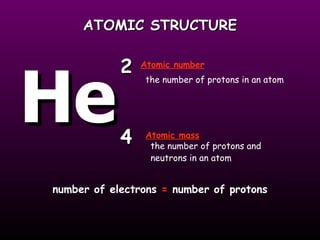
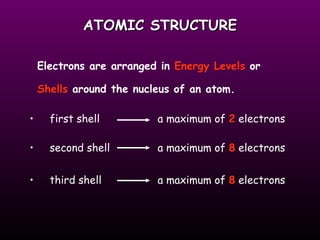
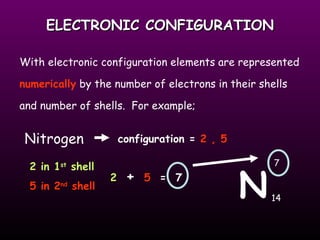
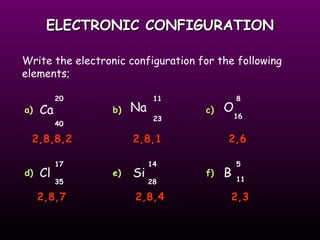
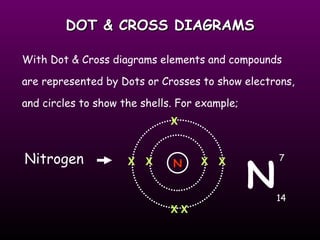
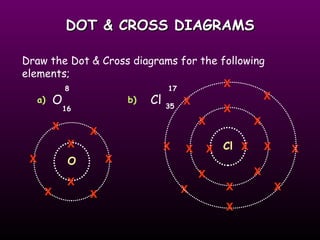
This document discusses the history and structure of the atomic model. It describes early theories from Democritus and Dalton that atoms were indivisible spheres. In the 1890s, Thomson discovered the electron and proposed the "plum pudding" model where electrons were distributed within the atom. Rutherford's gold foil experiment in 1910 showed that the positive charge and mass of atoms were concentrated in a small nucleus. Bohr added to this in 1913 by suggesting that electrons orbit the nucleus in set energy levels or shells. The document then explains atomic structure, including atomic number, mass, and how electrons are arranged in shells around the nucleus.