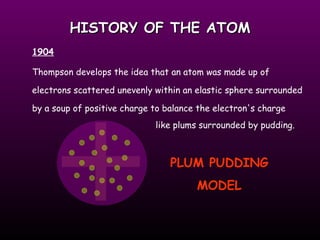
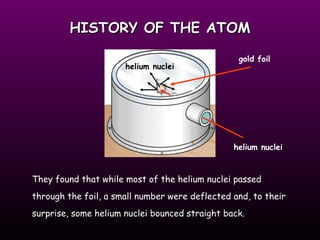
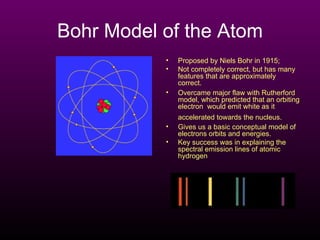
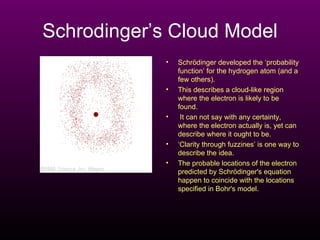
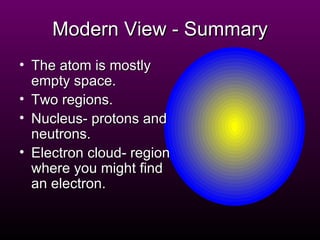
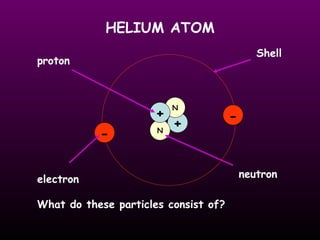
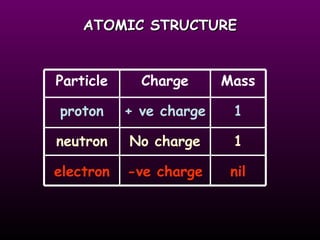
The document discusses the history of the atomic model from ancient Greek philosophers to modern theory. Democritus first proposed the idea of atoms in 460 BC. In the early 1900s, experiments by Thomson, Rutherford, and Bohr led to developments in the atomic model. Rutherford discovered the nucleus at the center of the atom in 1910. Bohr then proposed his atomic model in 1913 where electrons orbit the nucleus in defined shells. Later models like Schrodinger's probability cloud refined and improved upon understanding of atomic structure. The modern view is that the atom is mostly empty space with a dense nucleus at the center and electron cloud region.