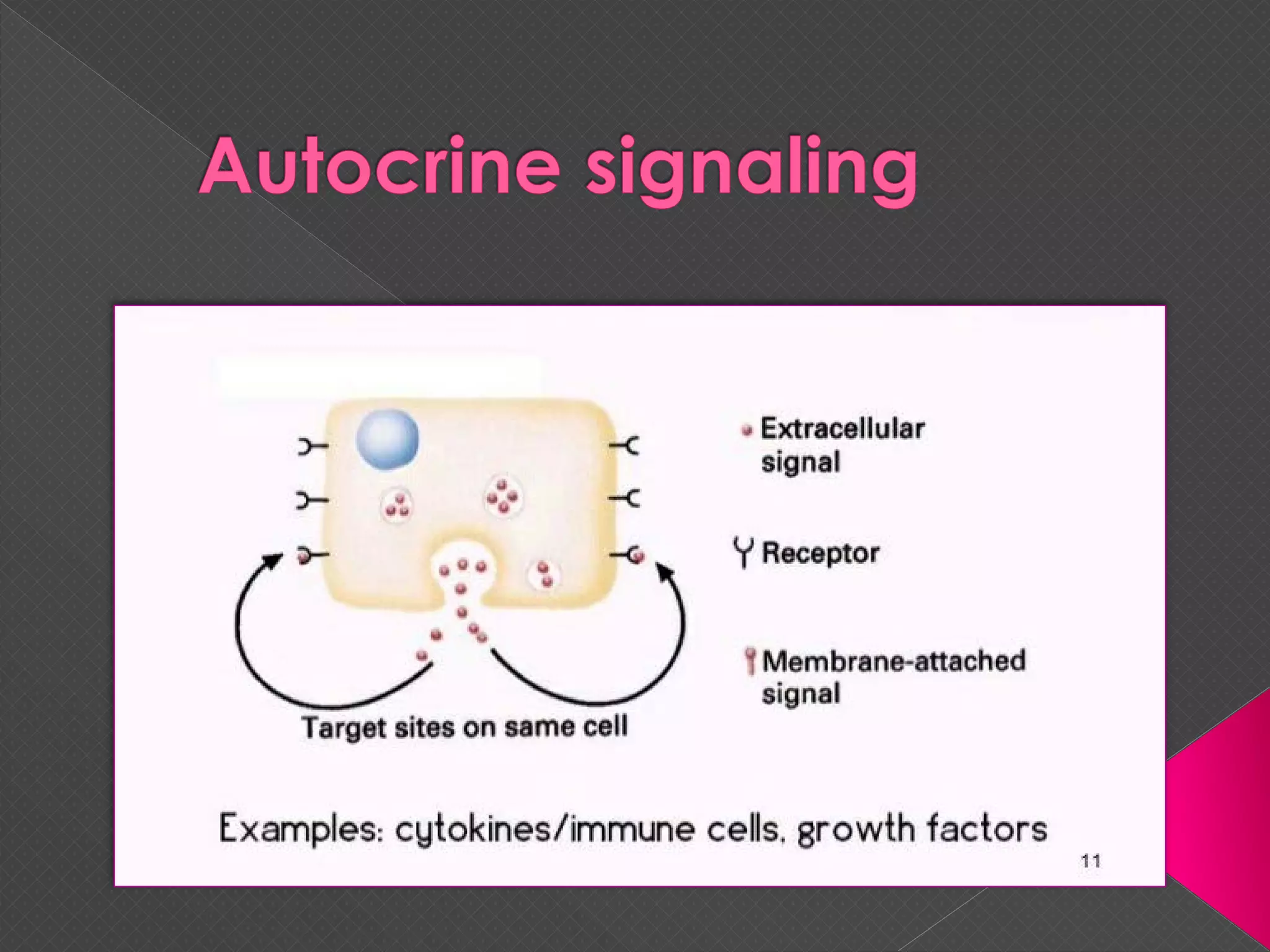
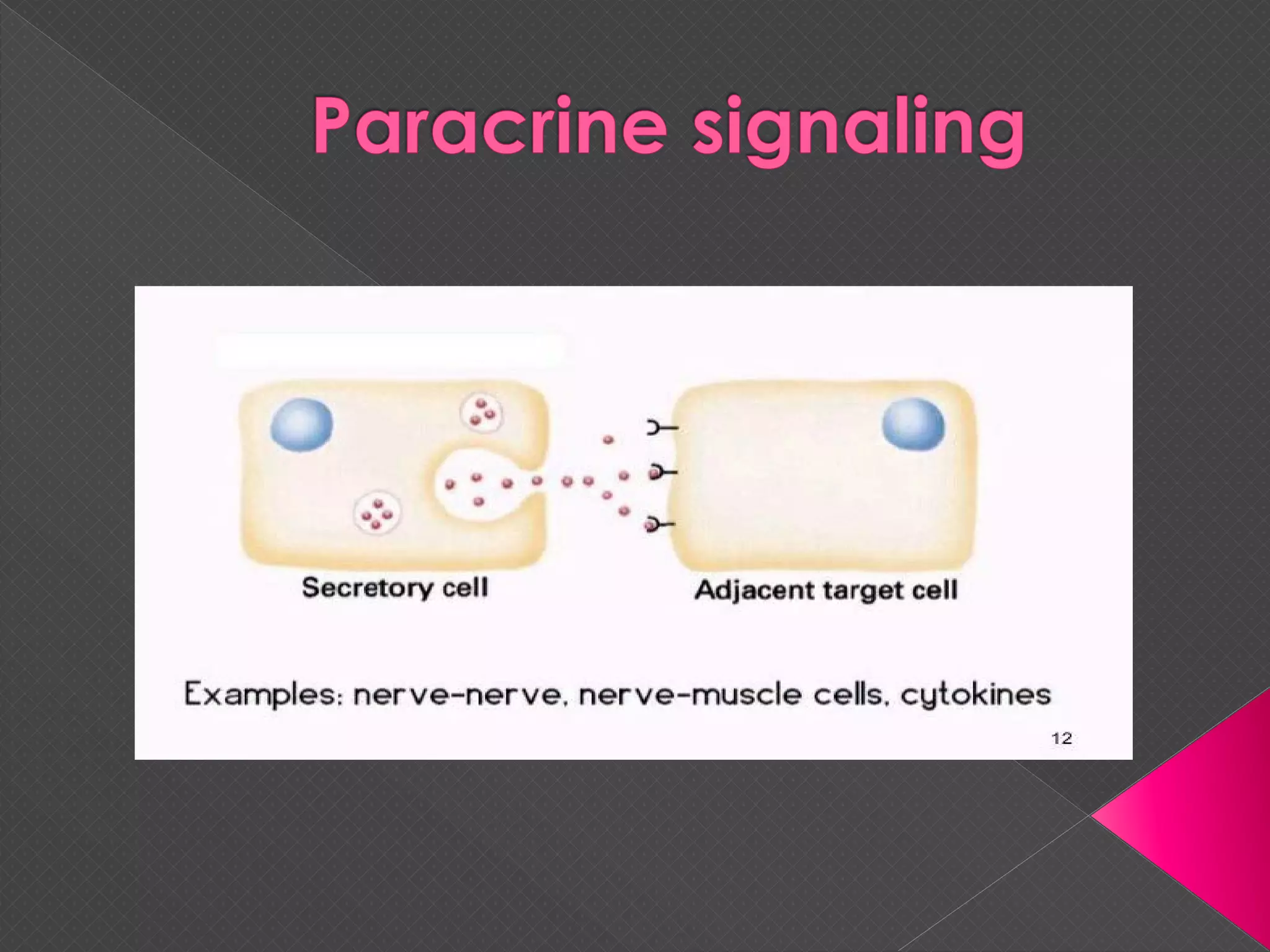
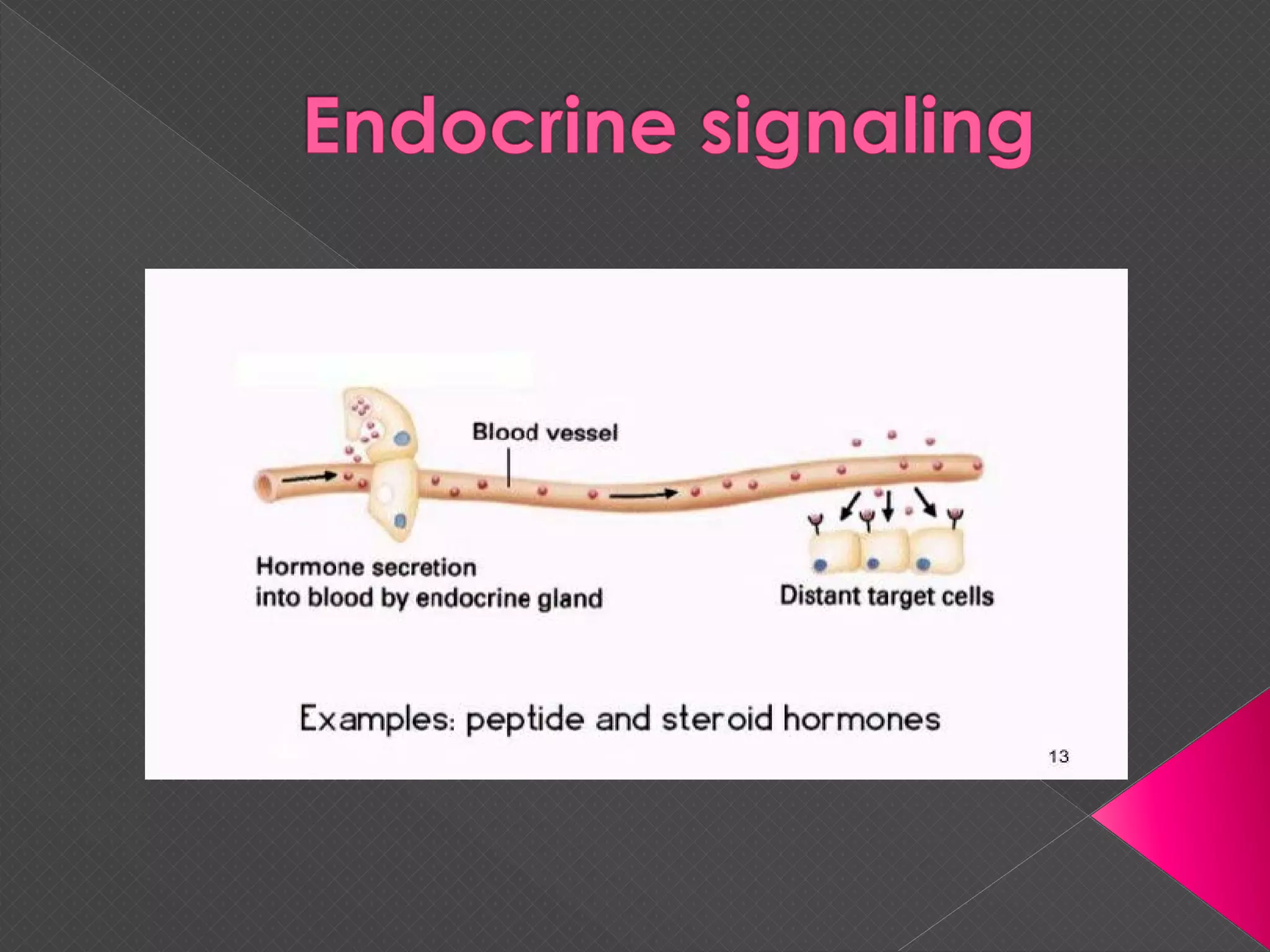
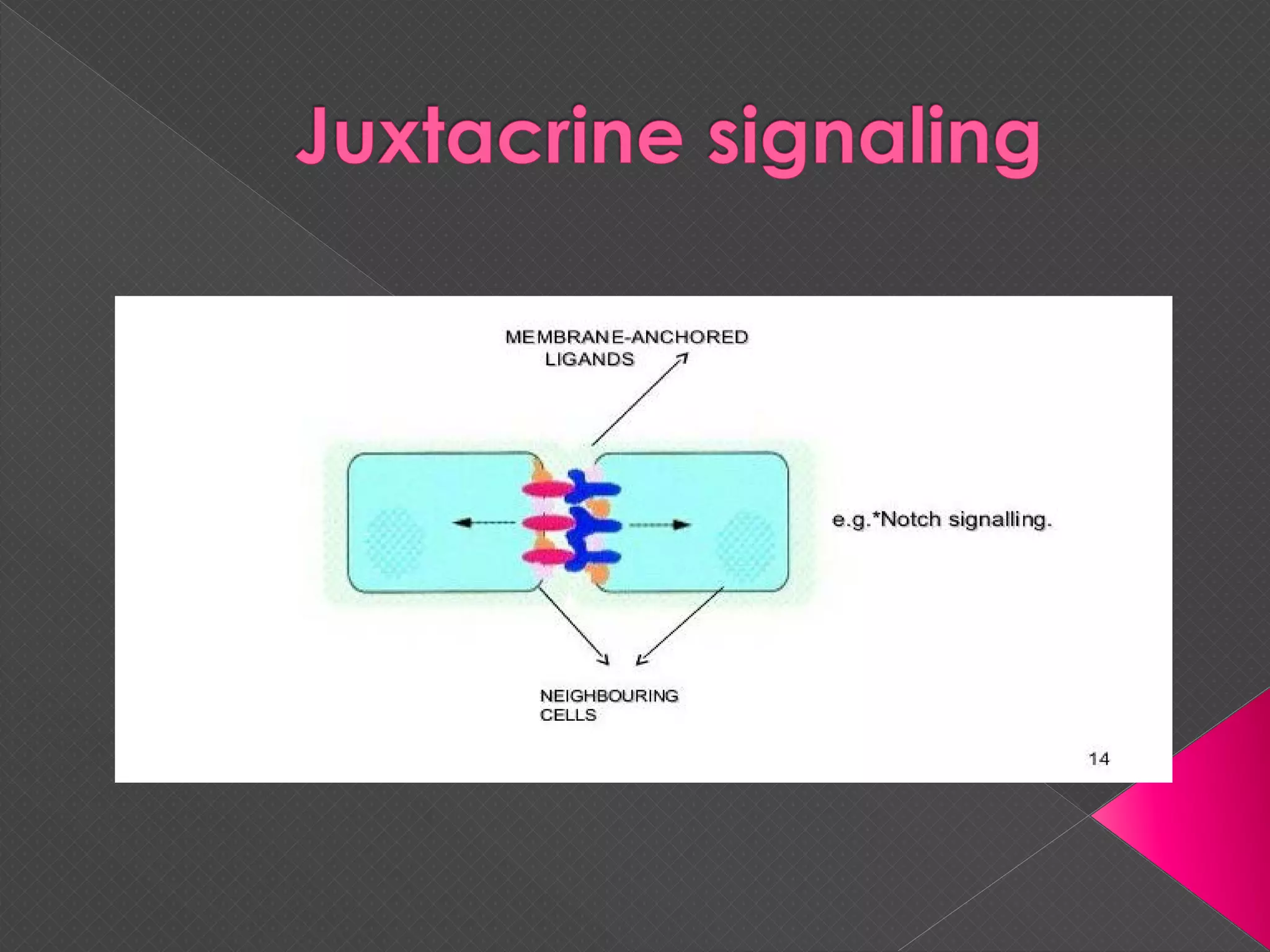
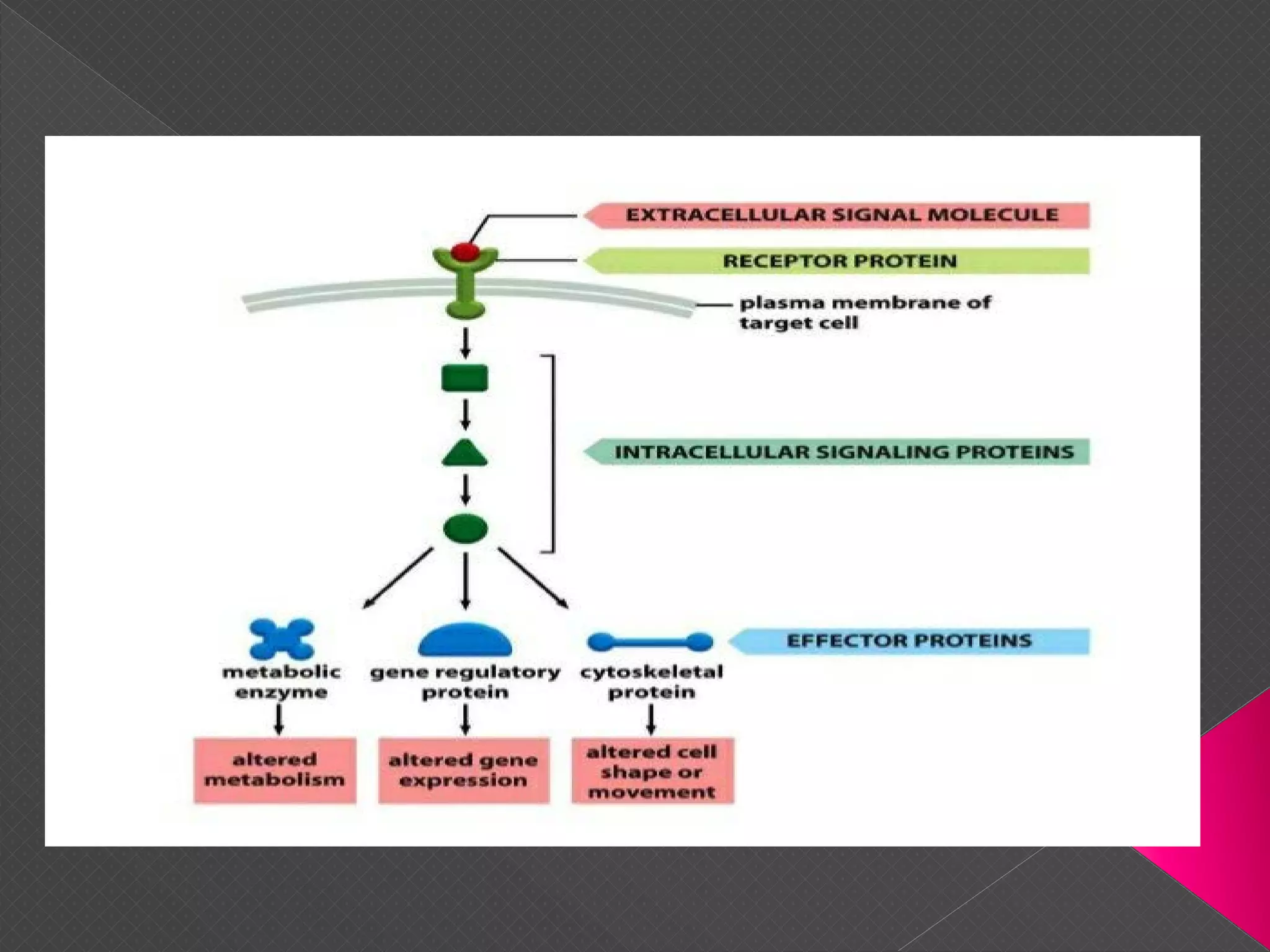
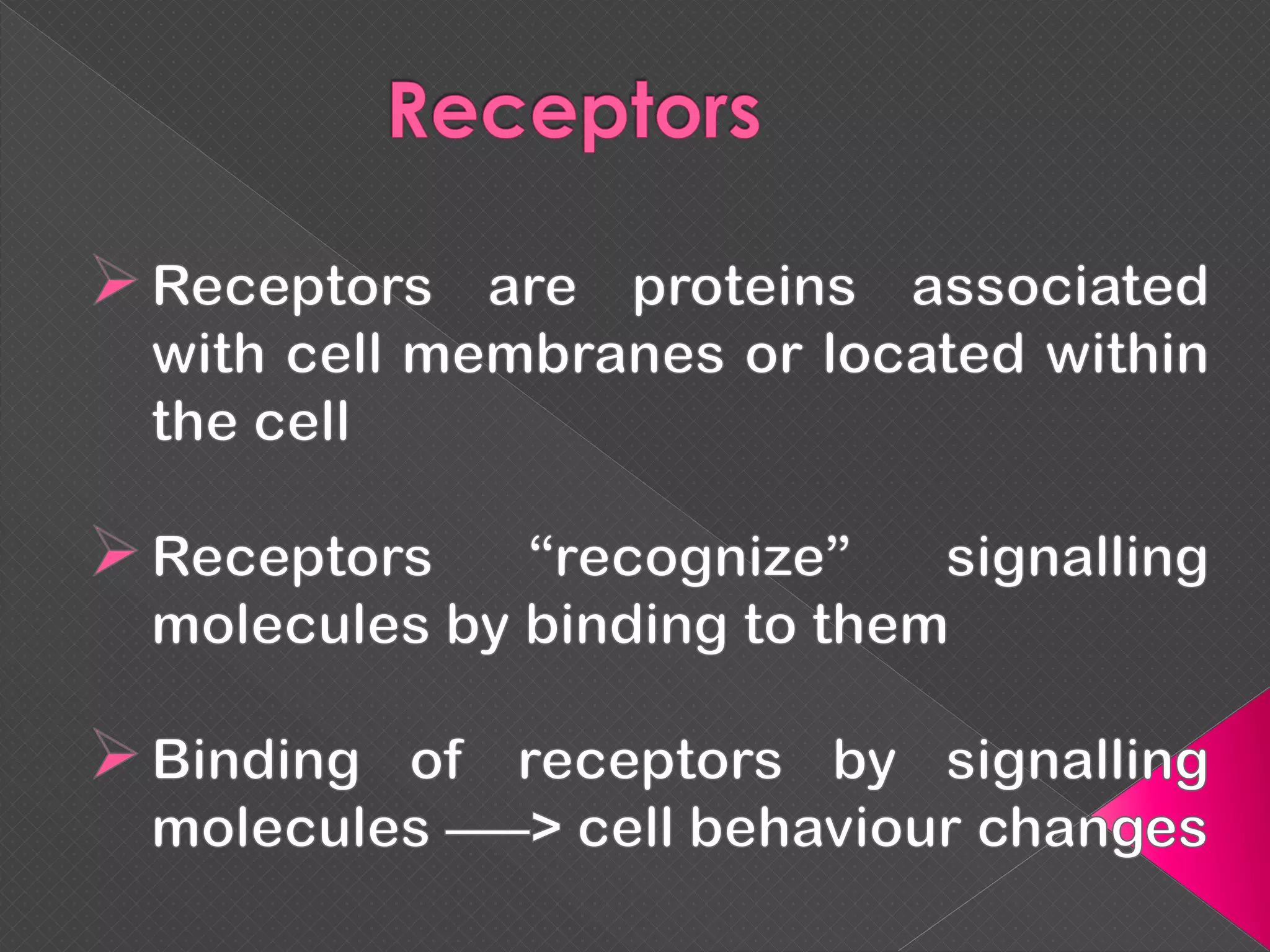
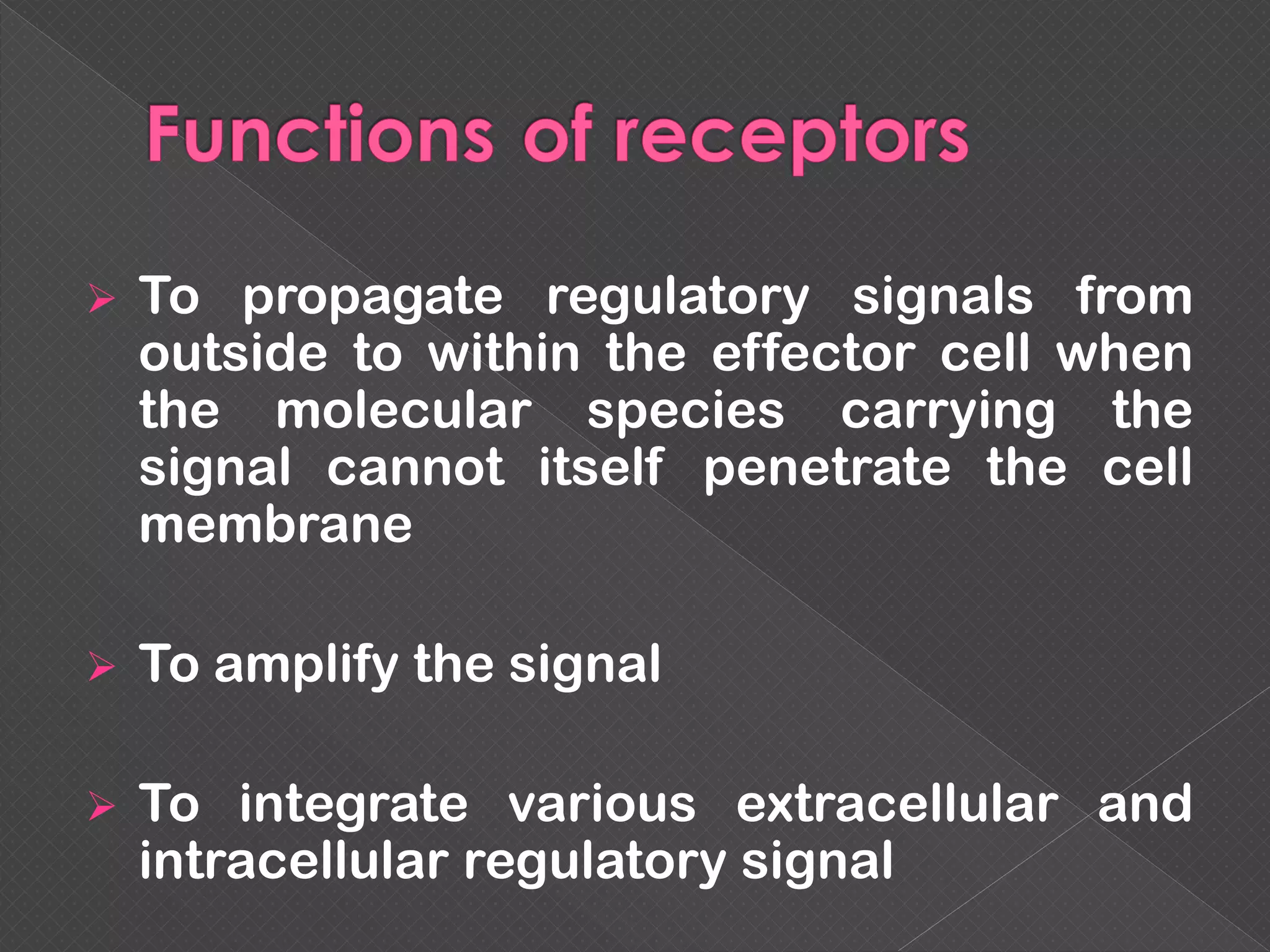
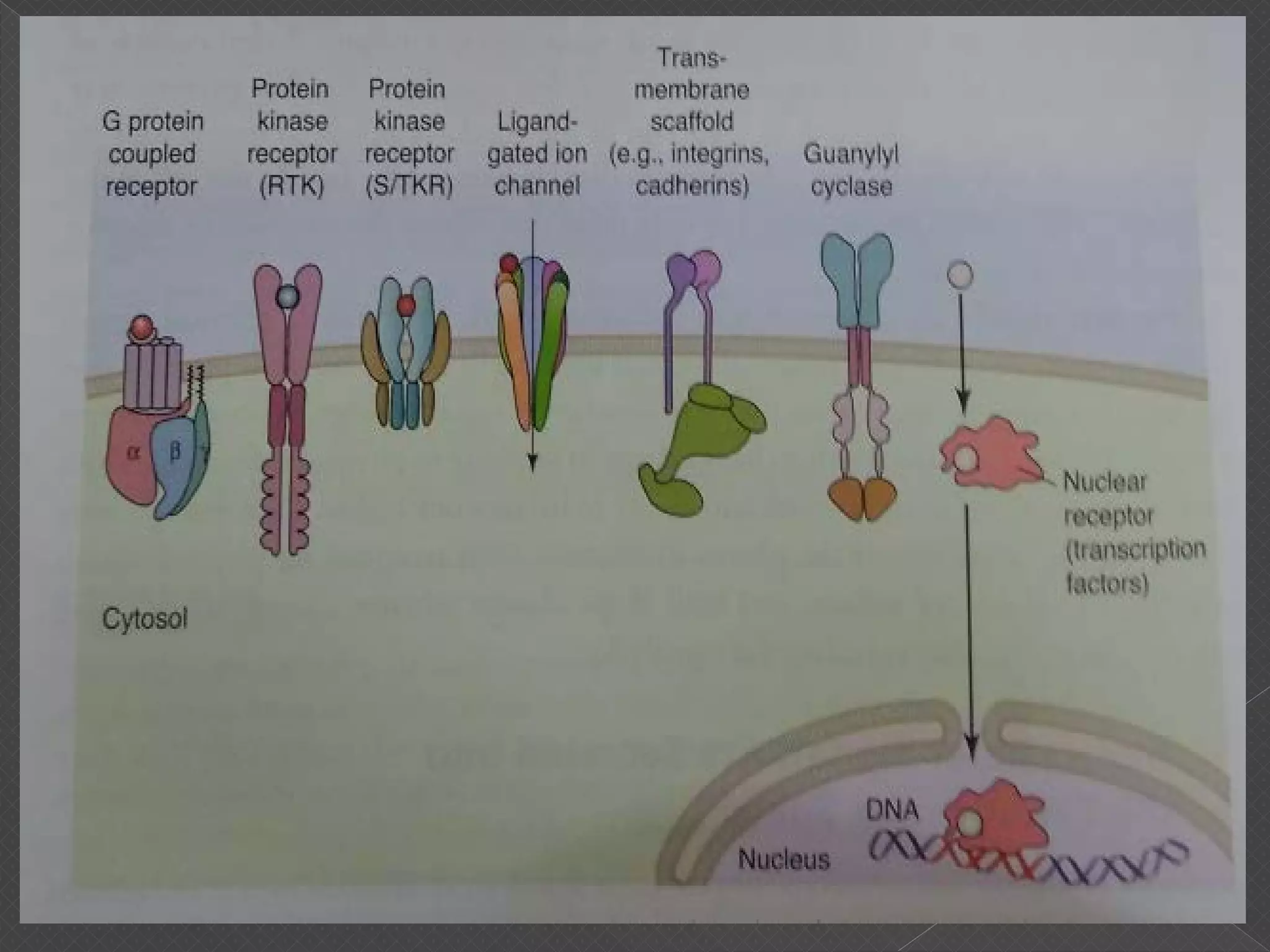
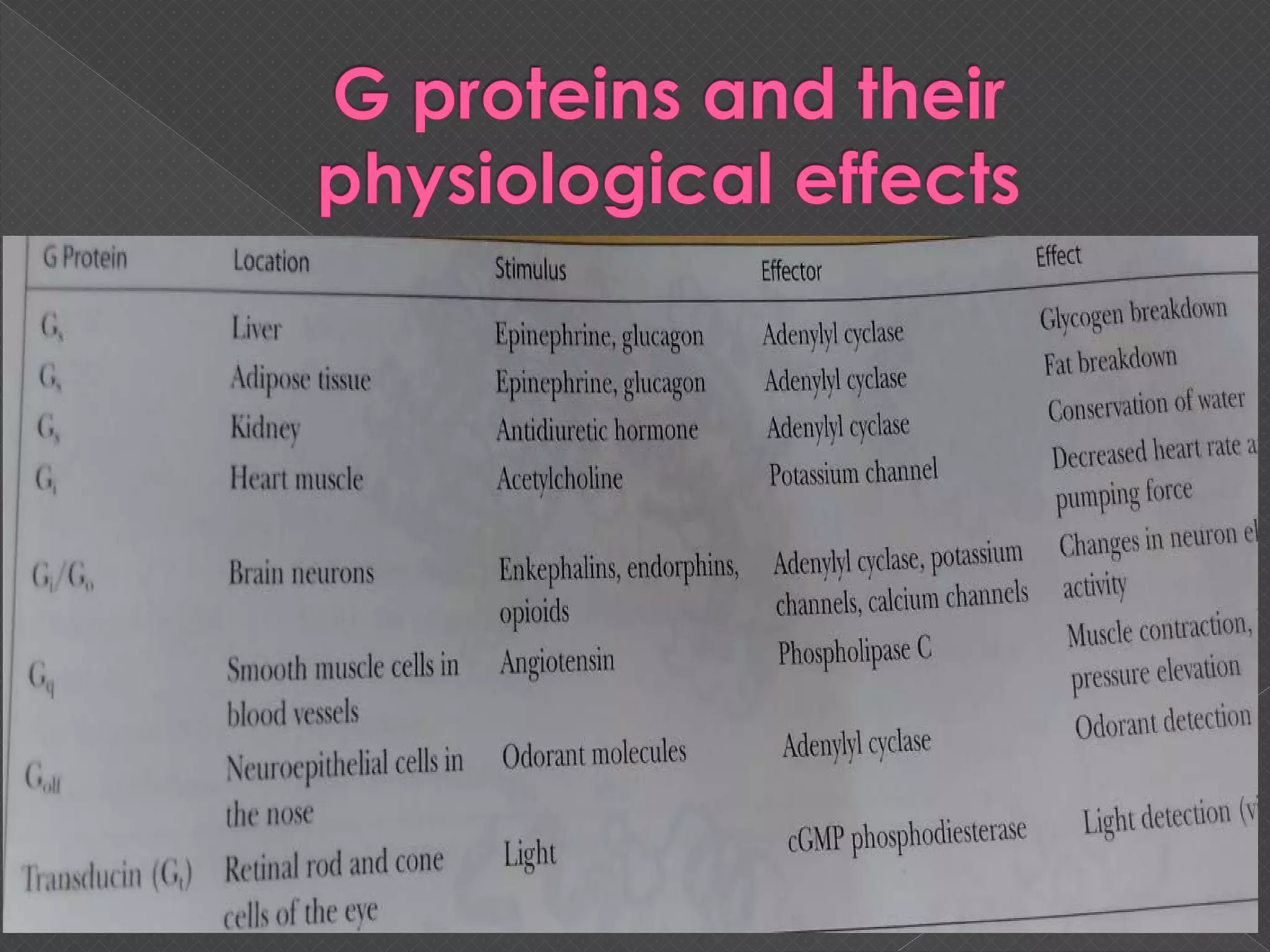
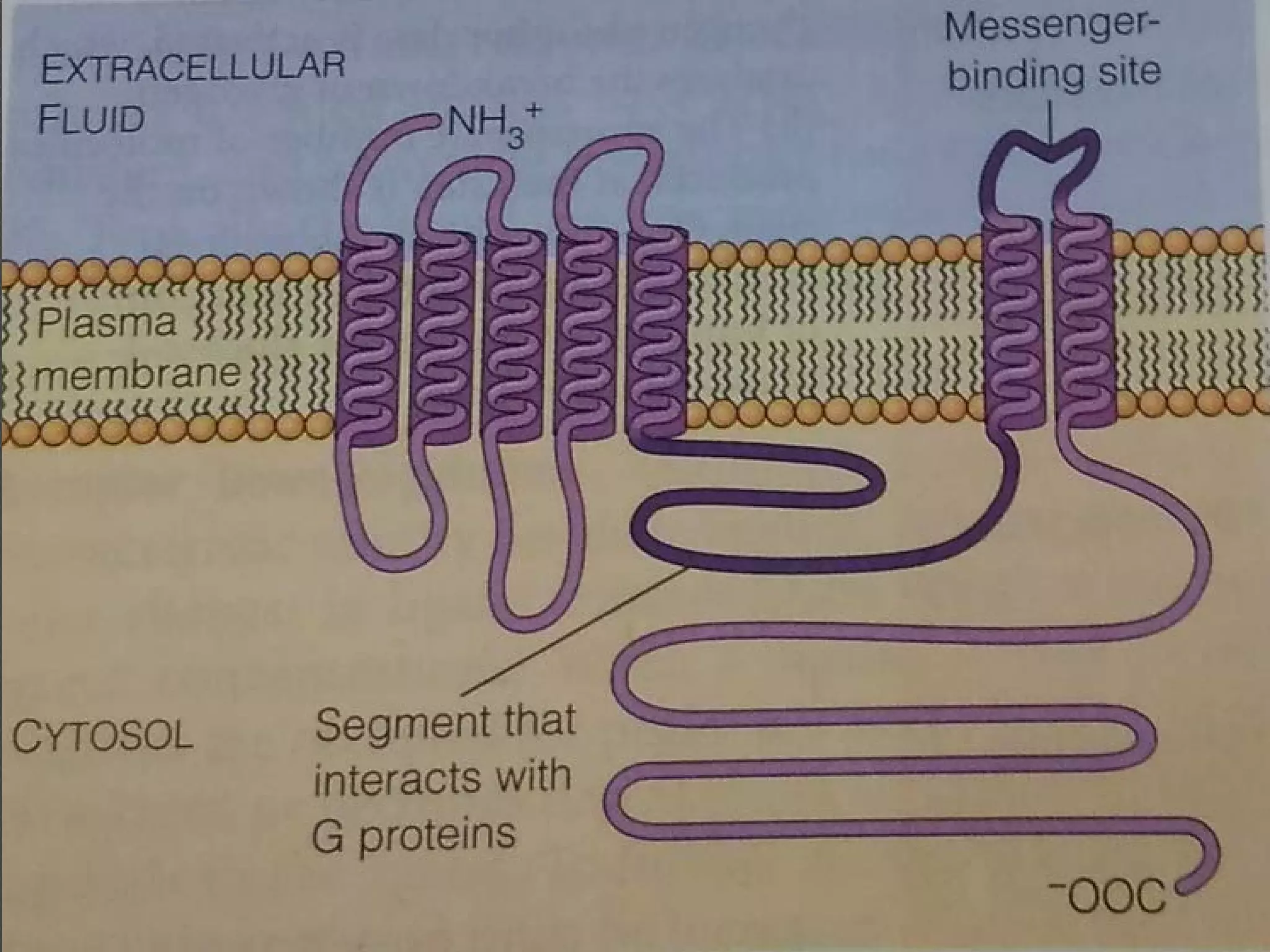
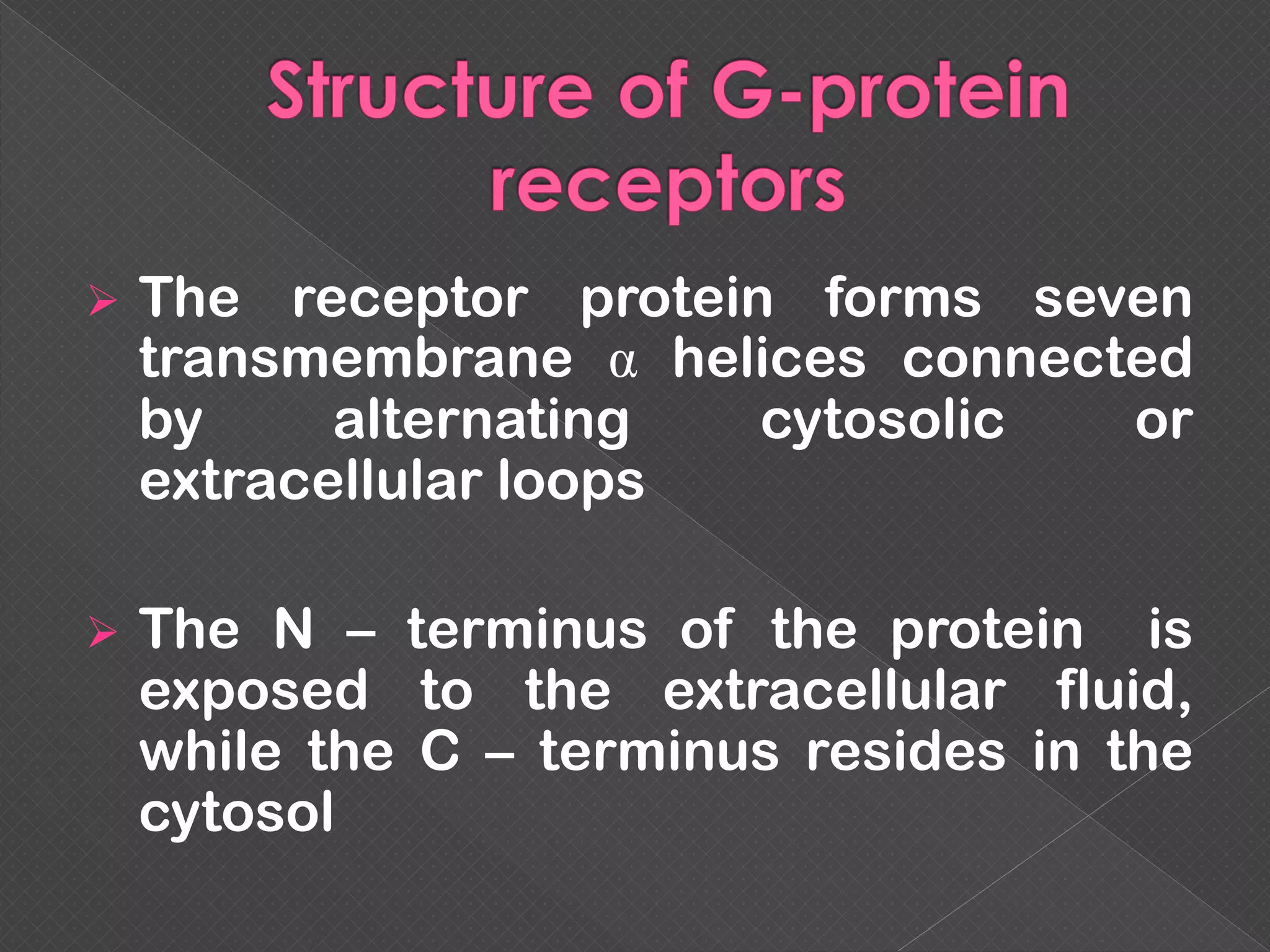
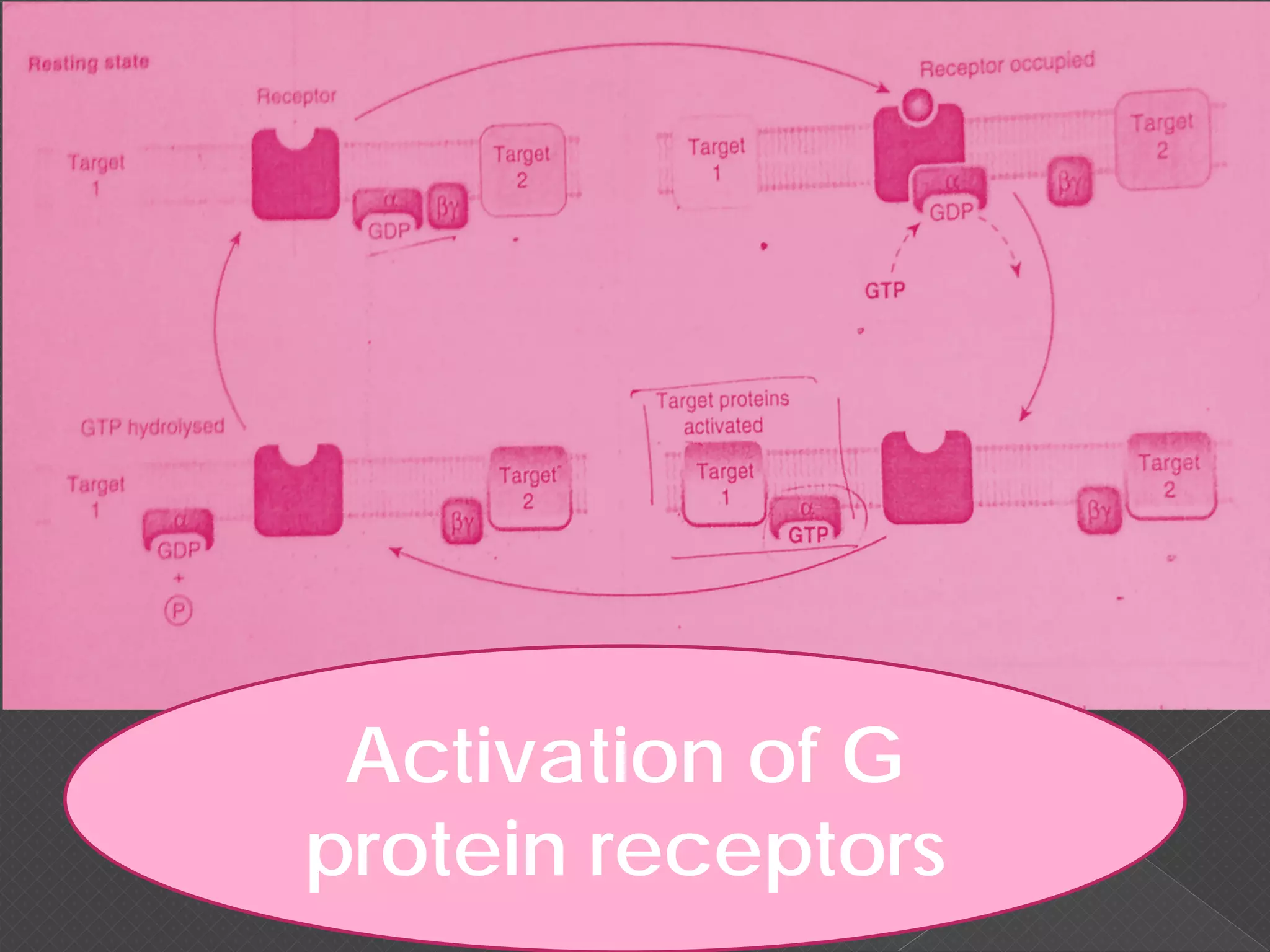
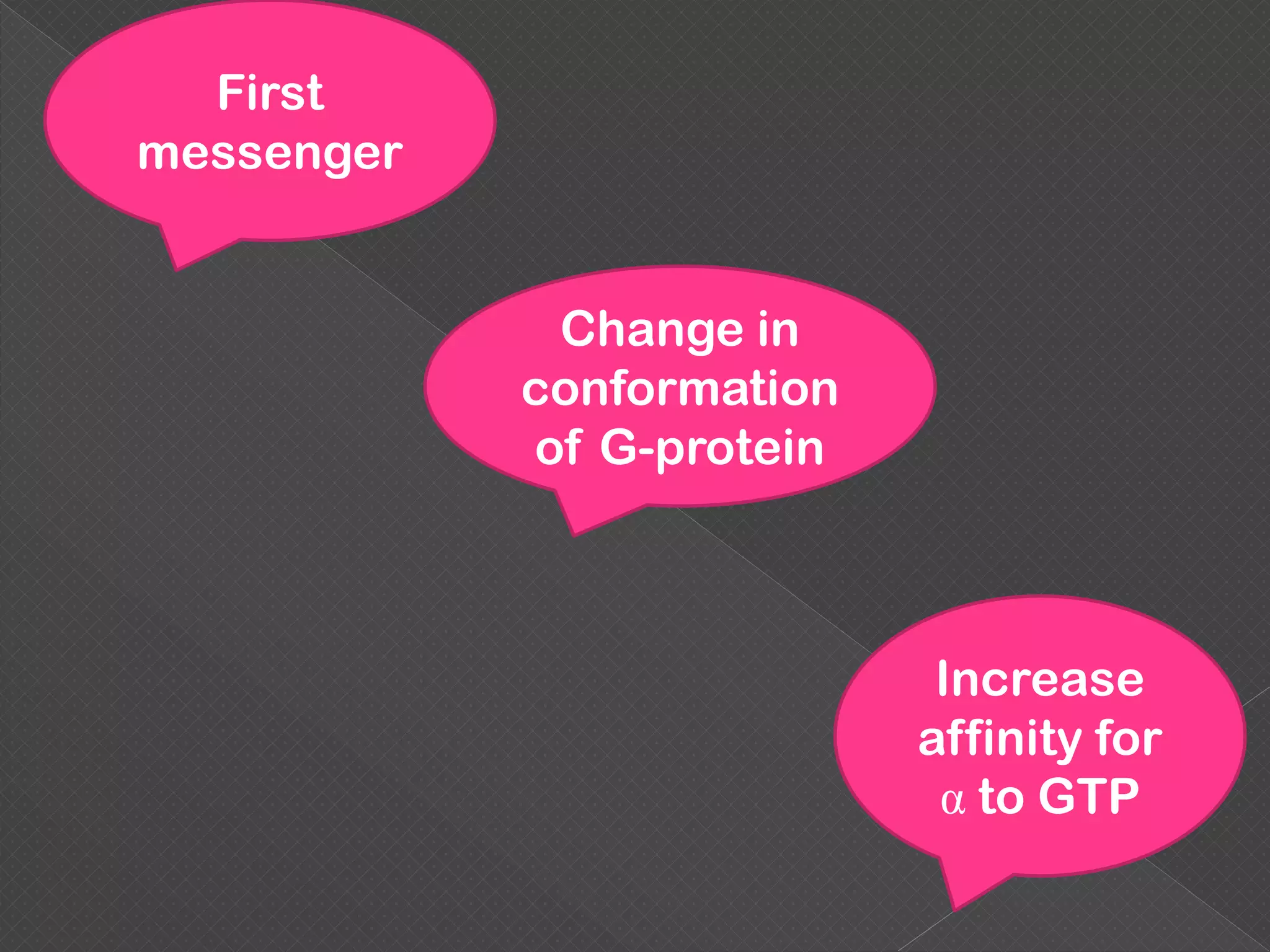
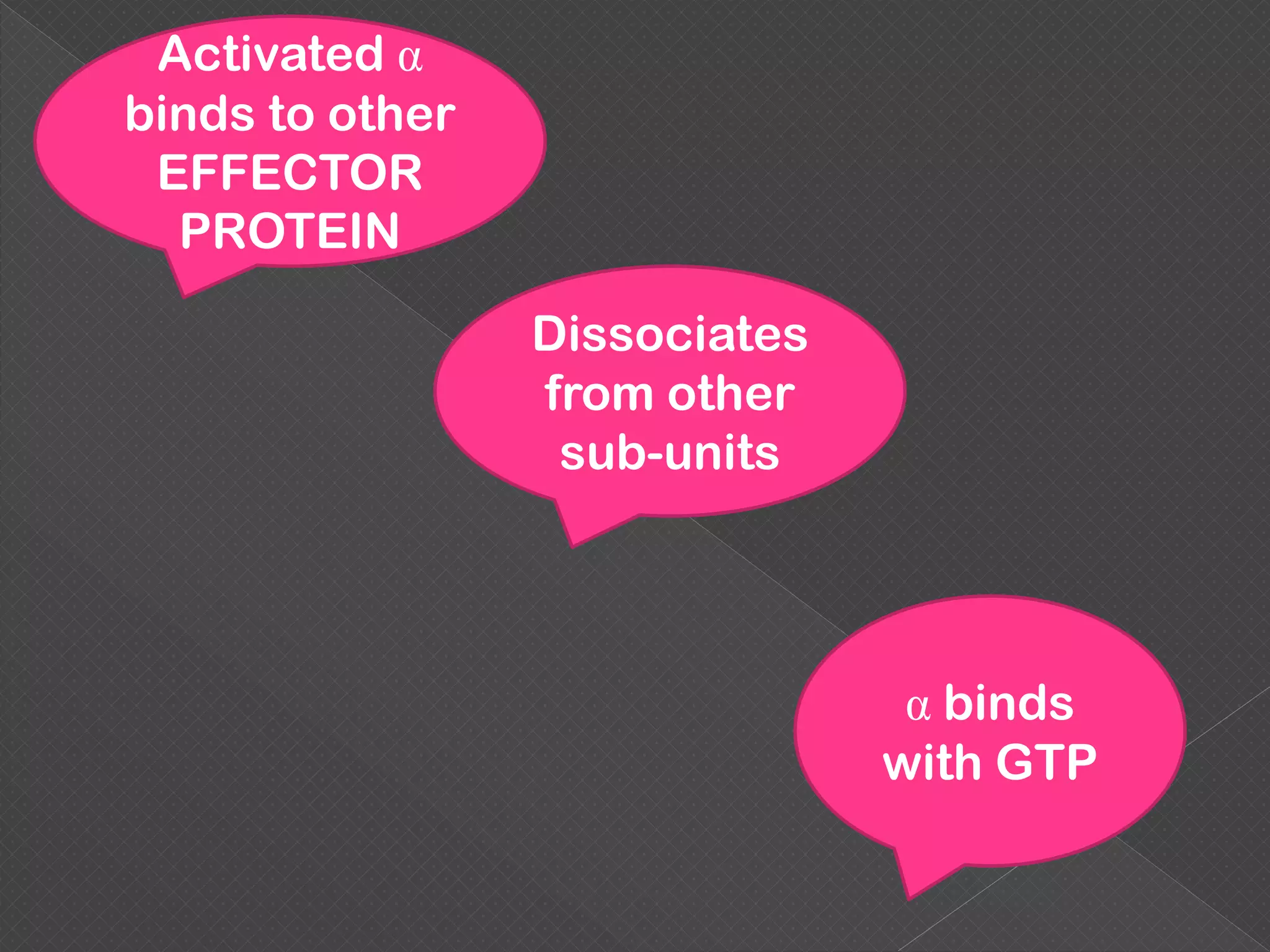
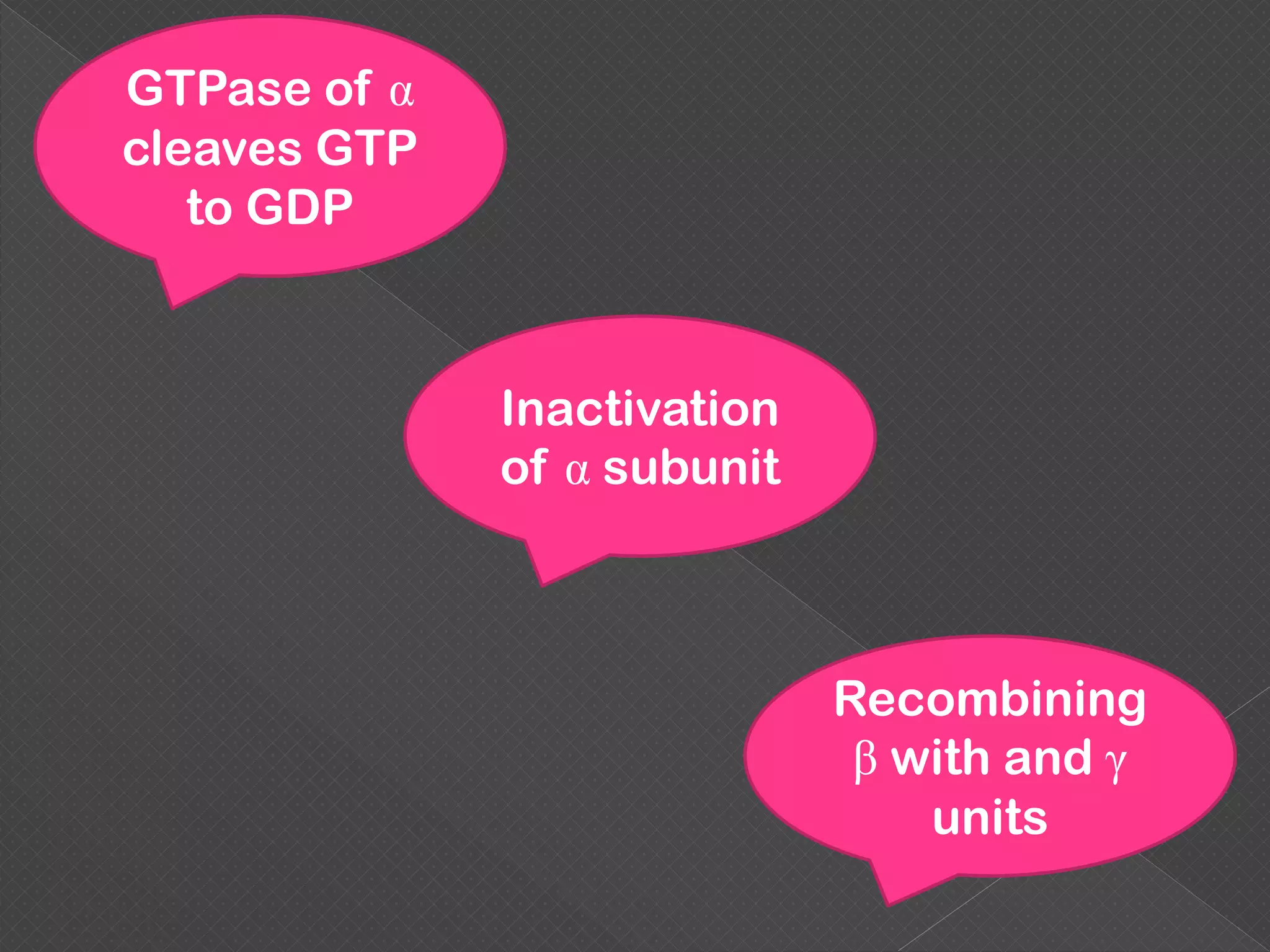
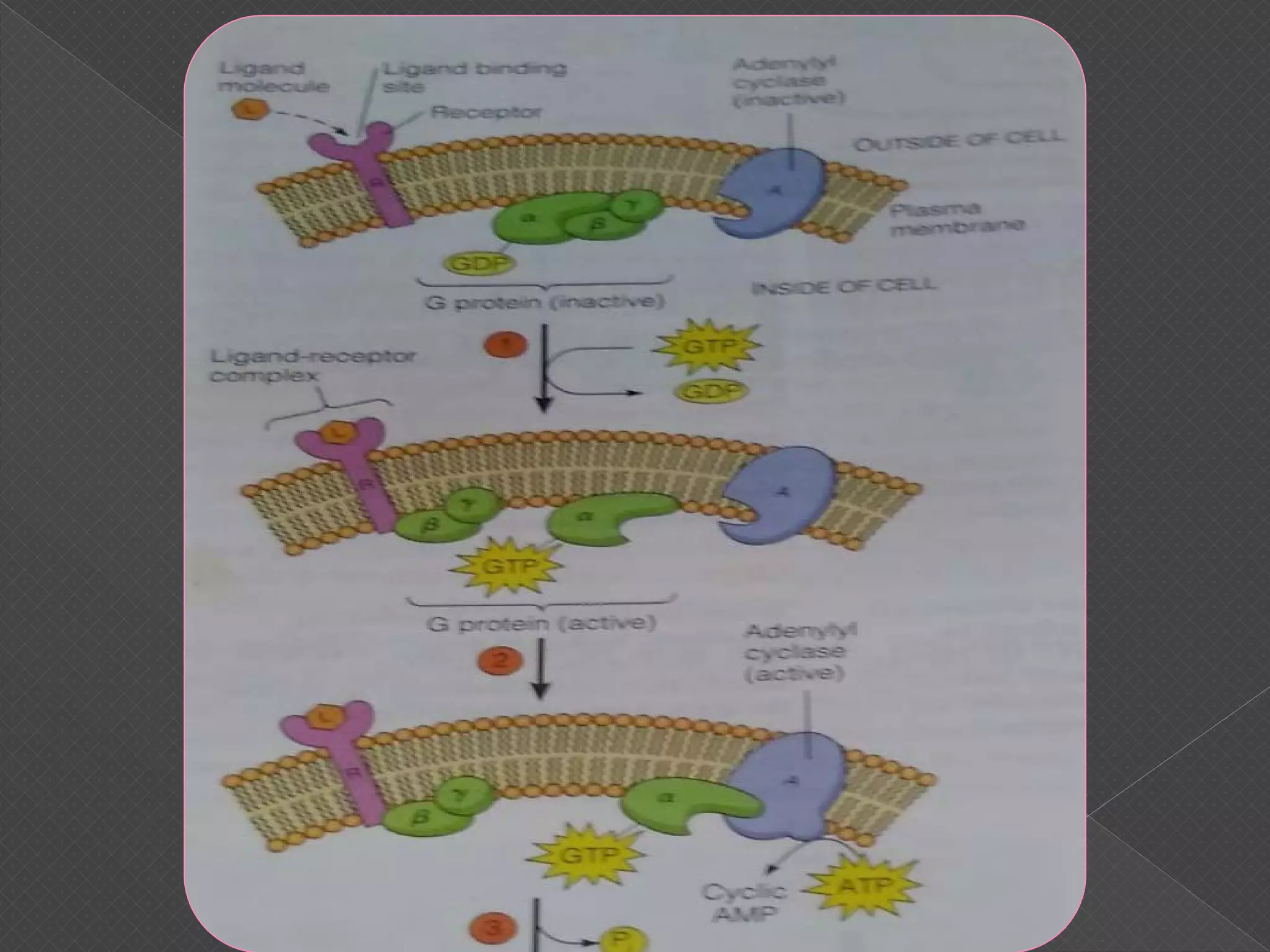
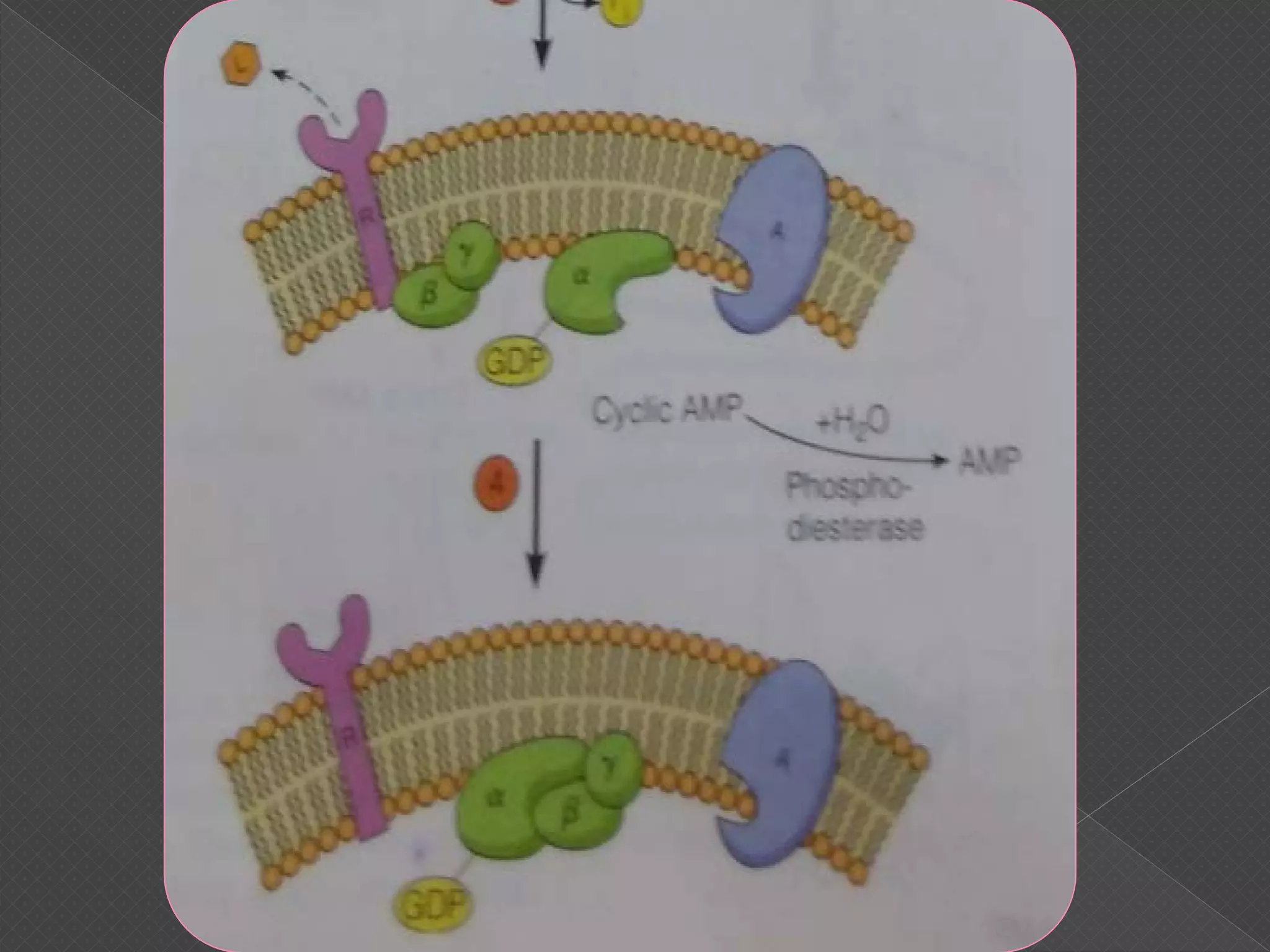
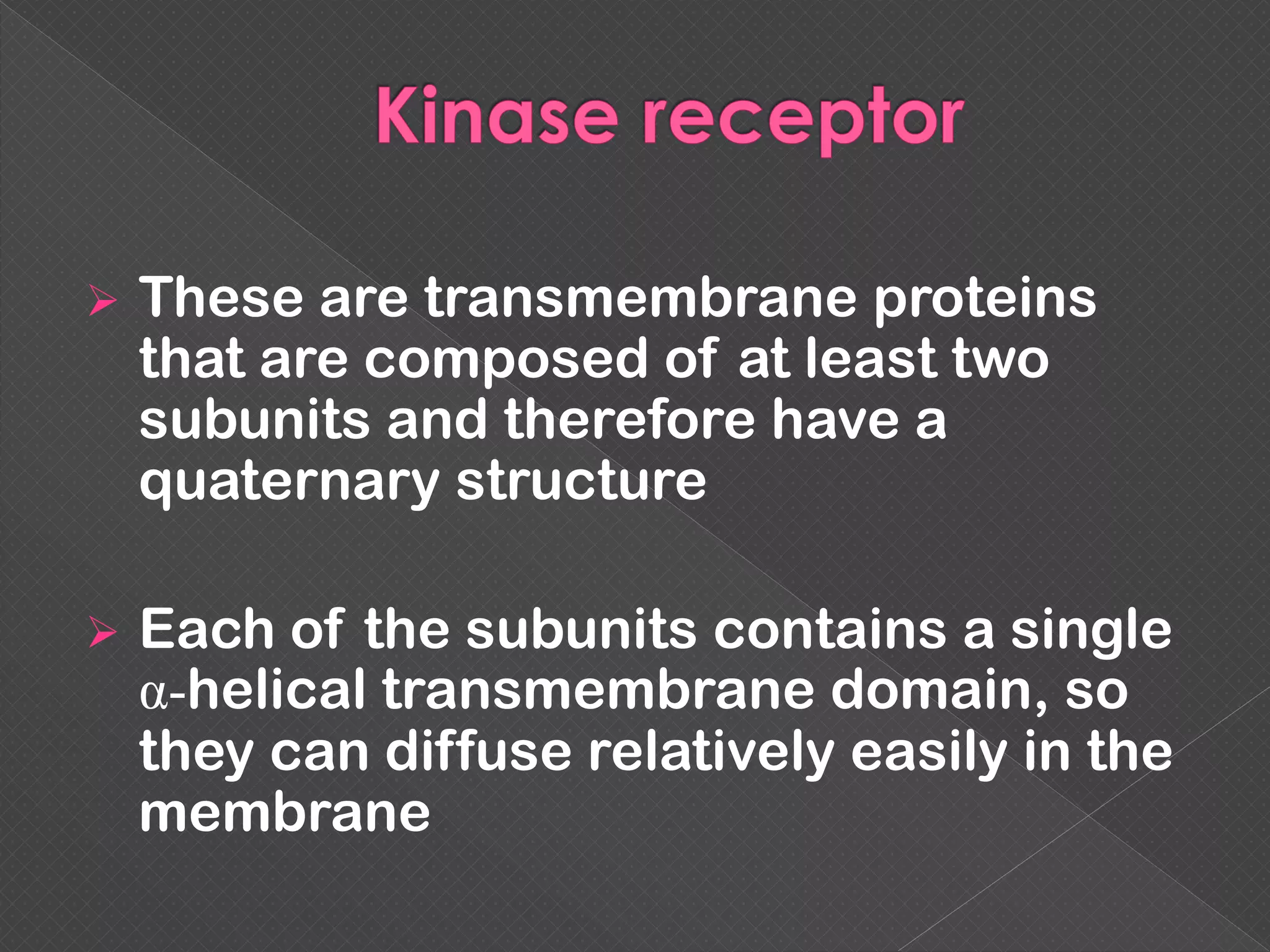
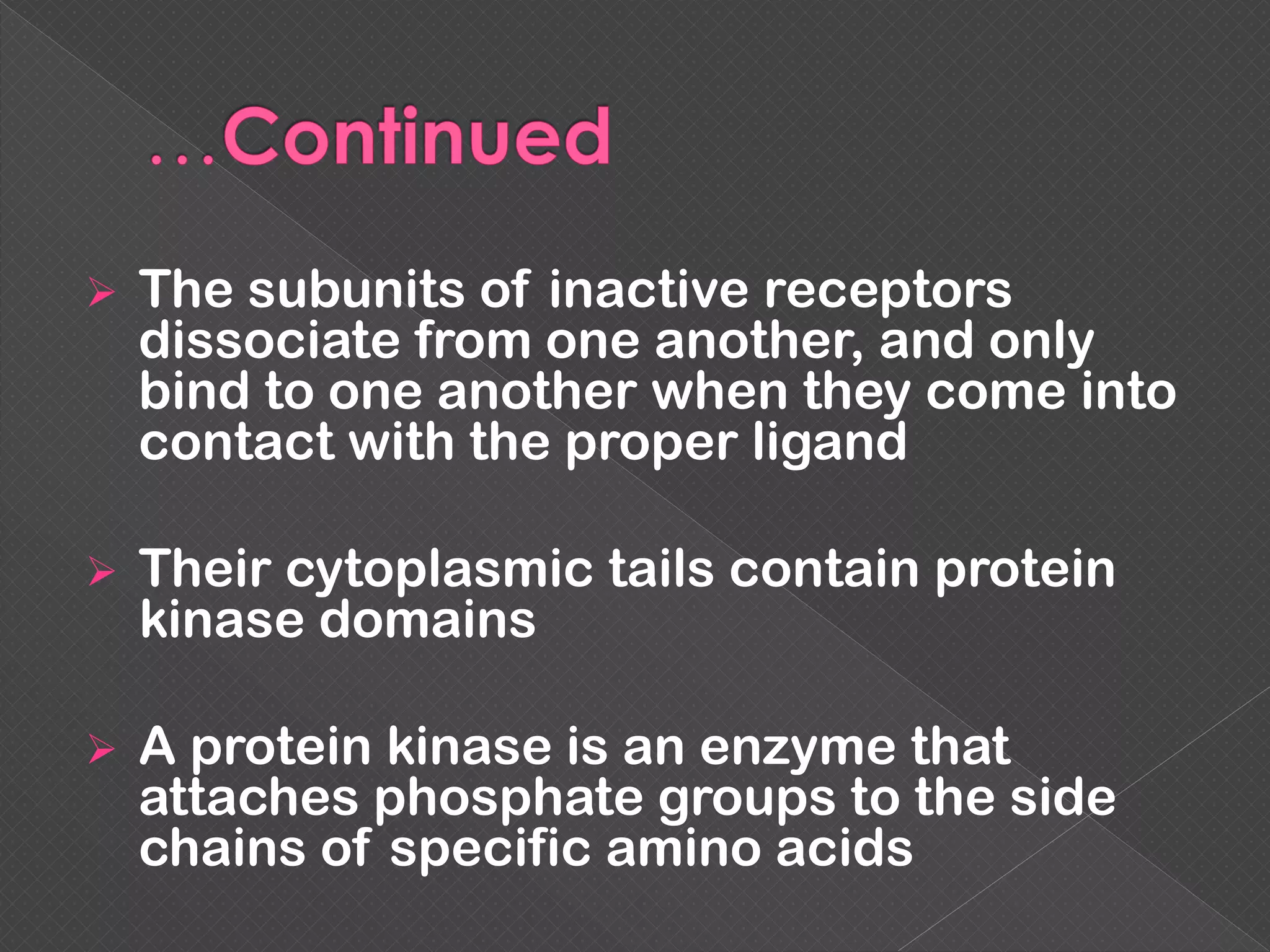
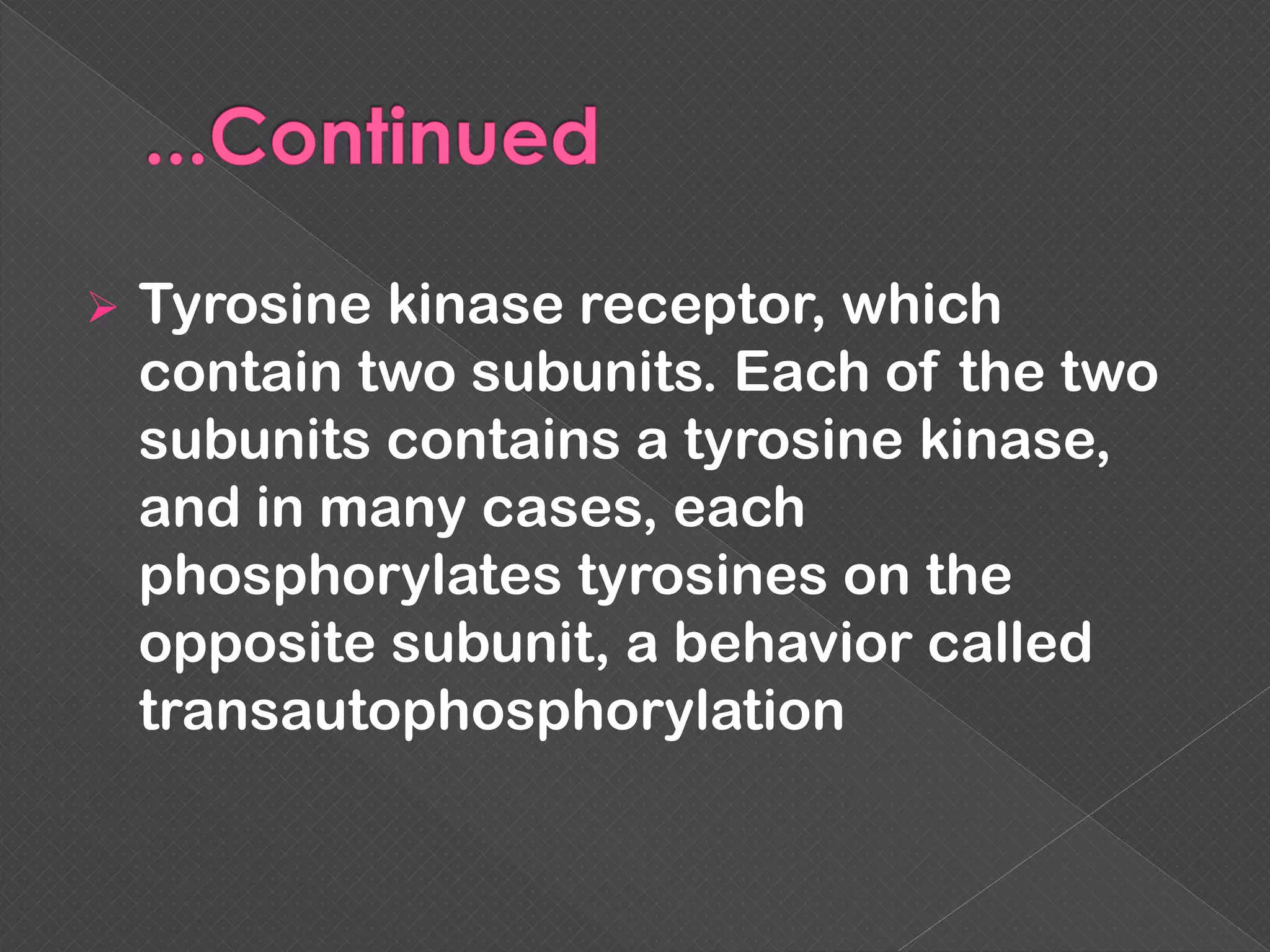
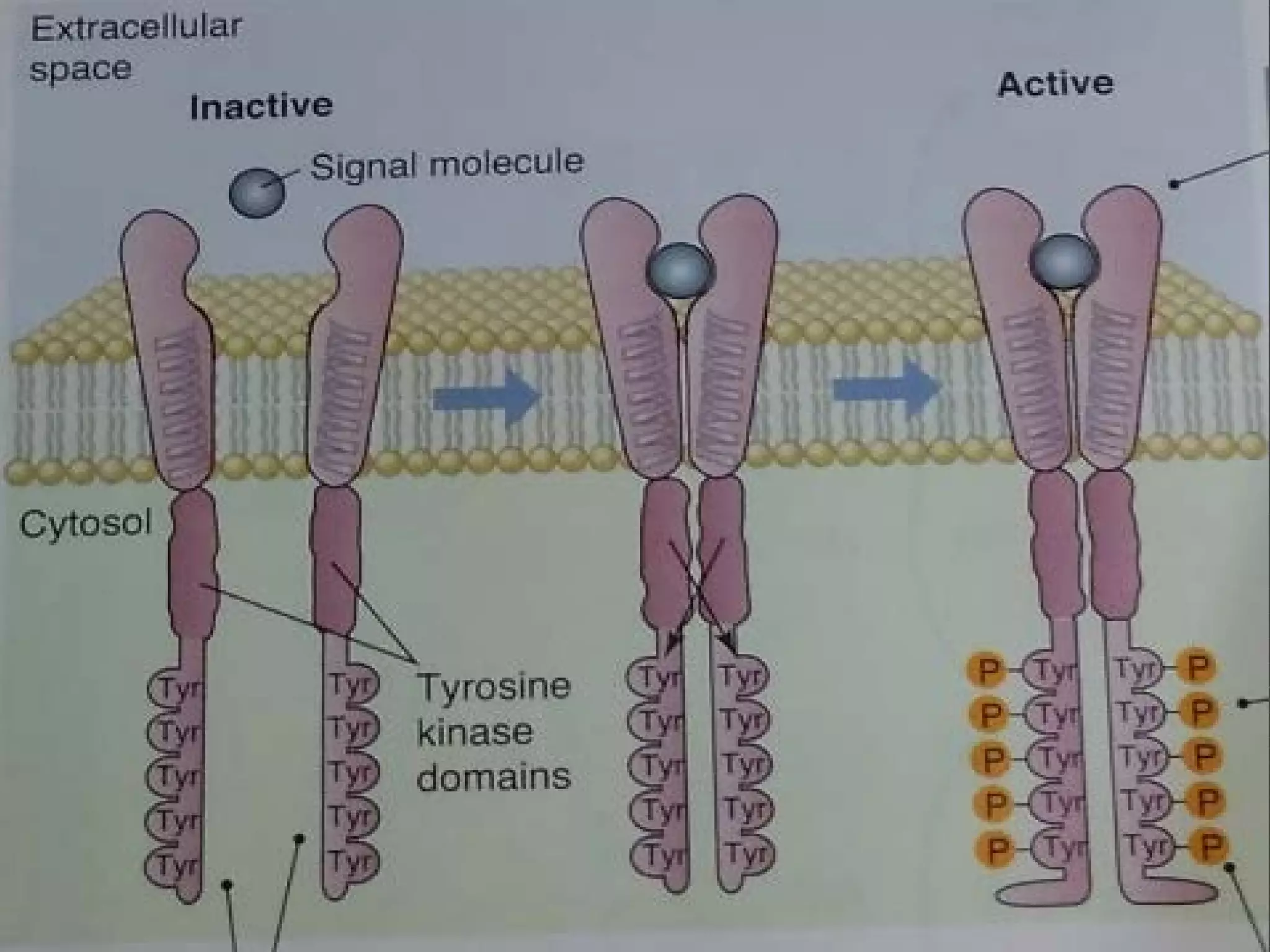
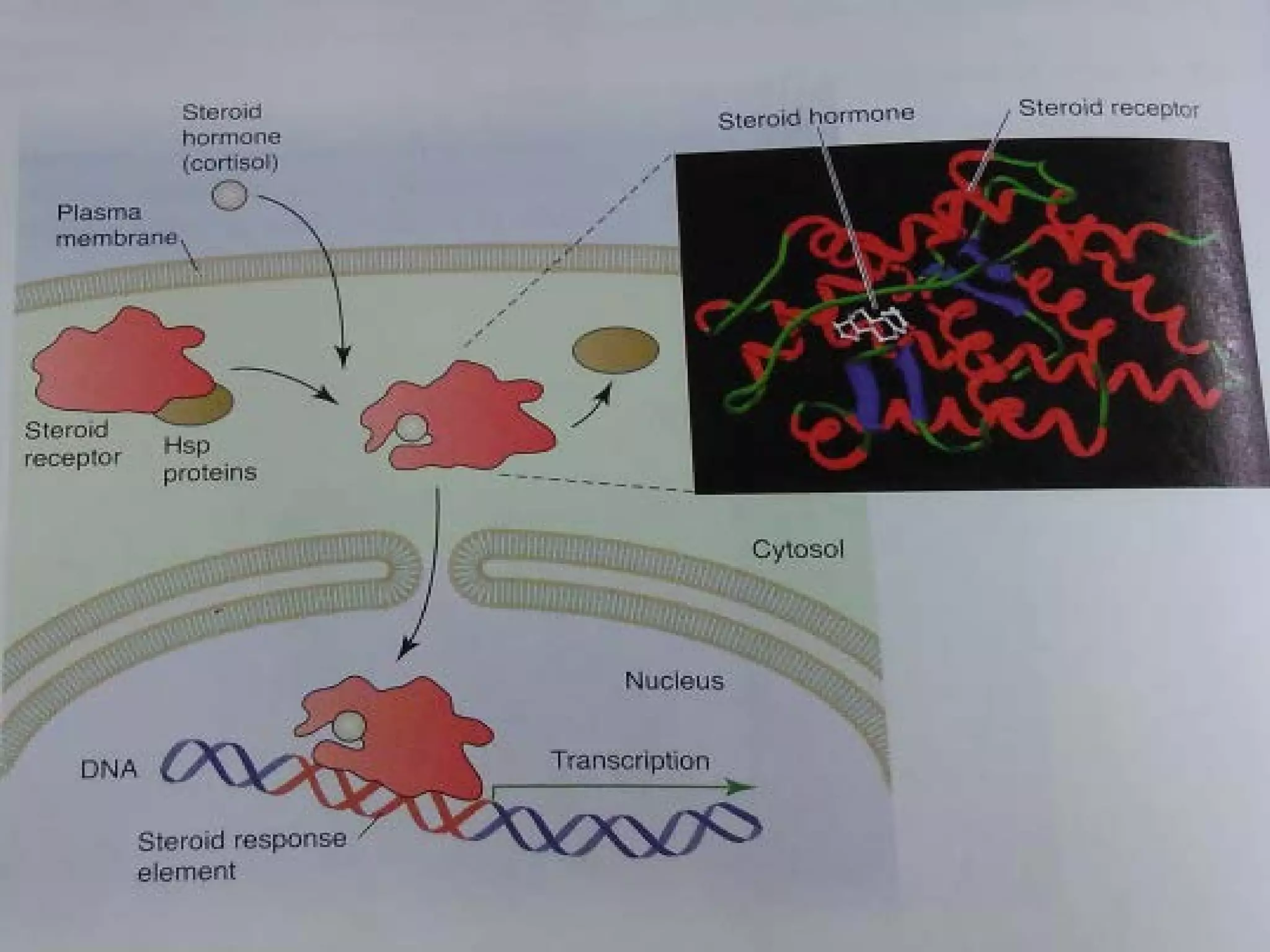
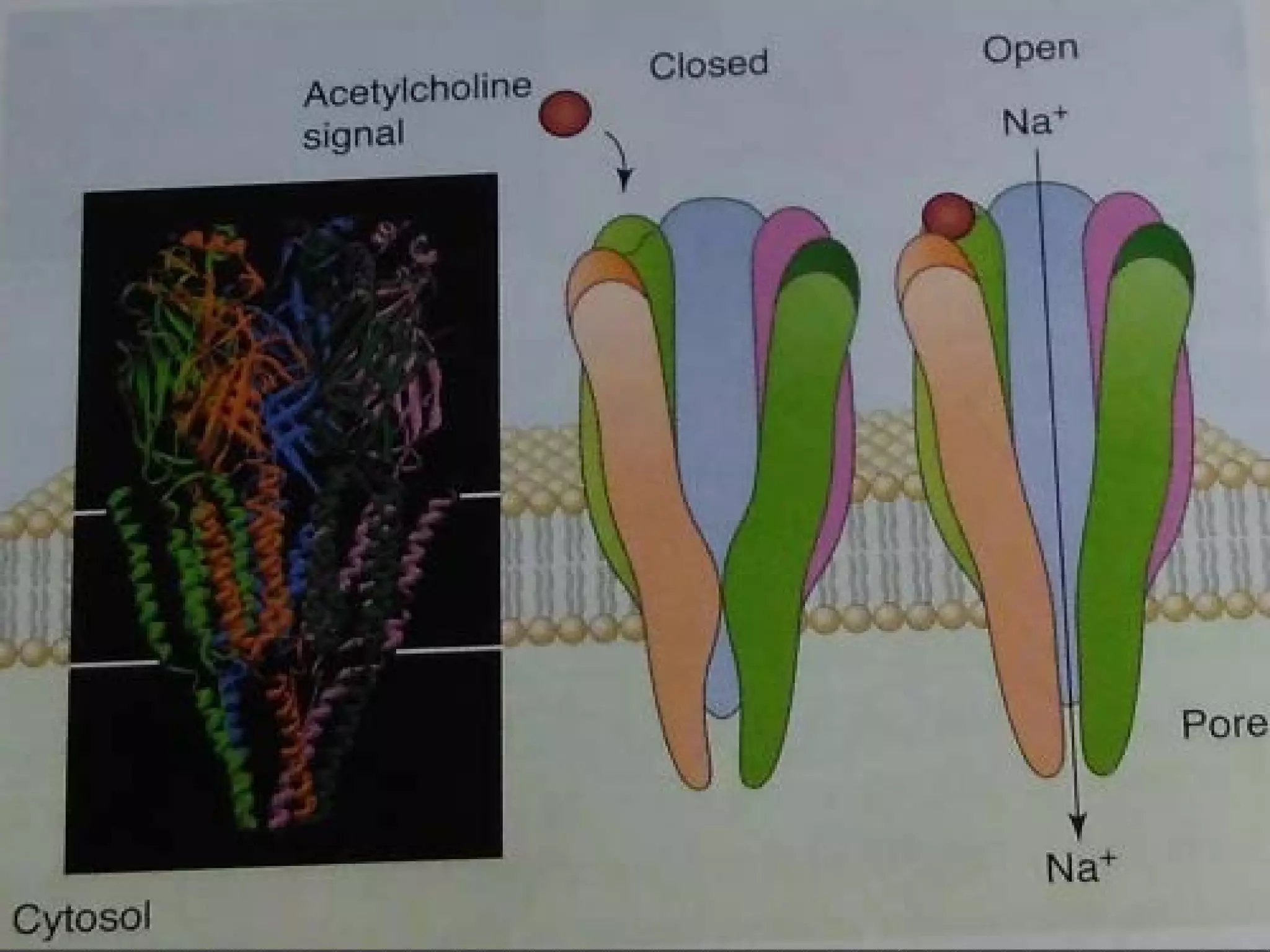
The document provides an overview of various signaling mechanisms in cells, including autocrine, paracrine, endocrine, and juxtacrine signaling. It discusses G protein-coupled receptors, receptor tyrosine kinases, and ionotropic receptors, detailing their structures, functions, and roles in signal transduction processes. Additionally, it highlights how these signaling pathways impact cellular responses and activities, such as growth and differentiation.