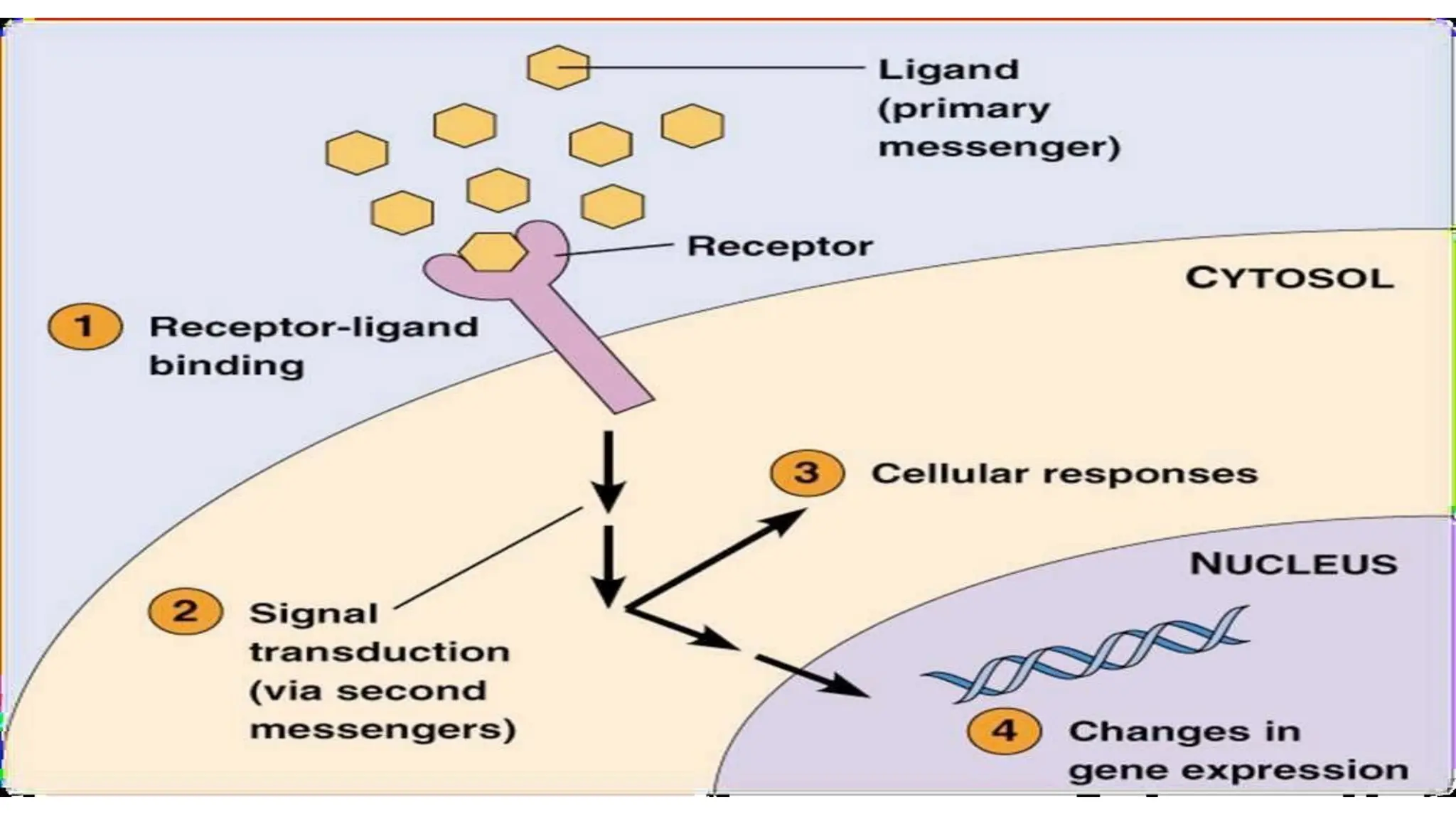
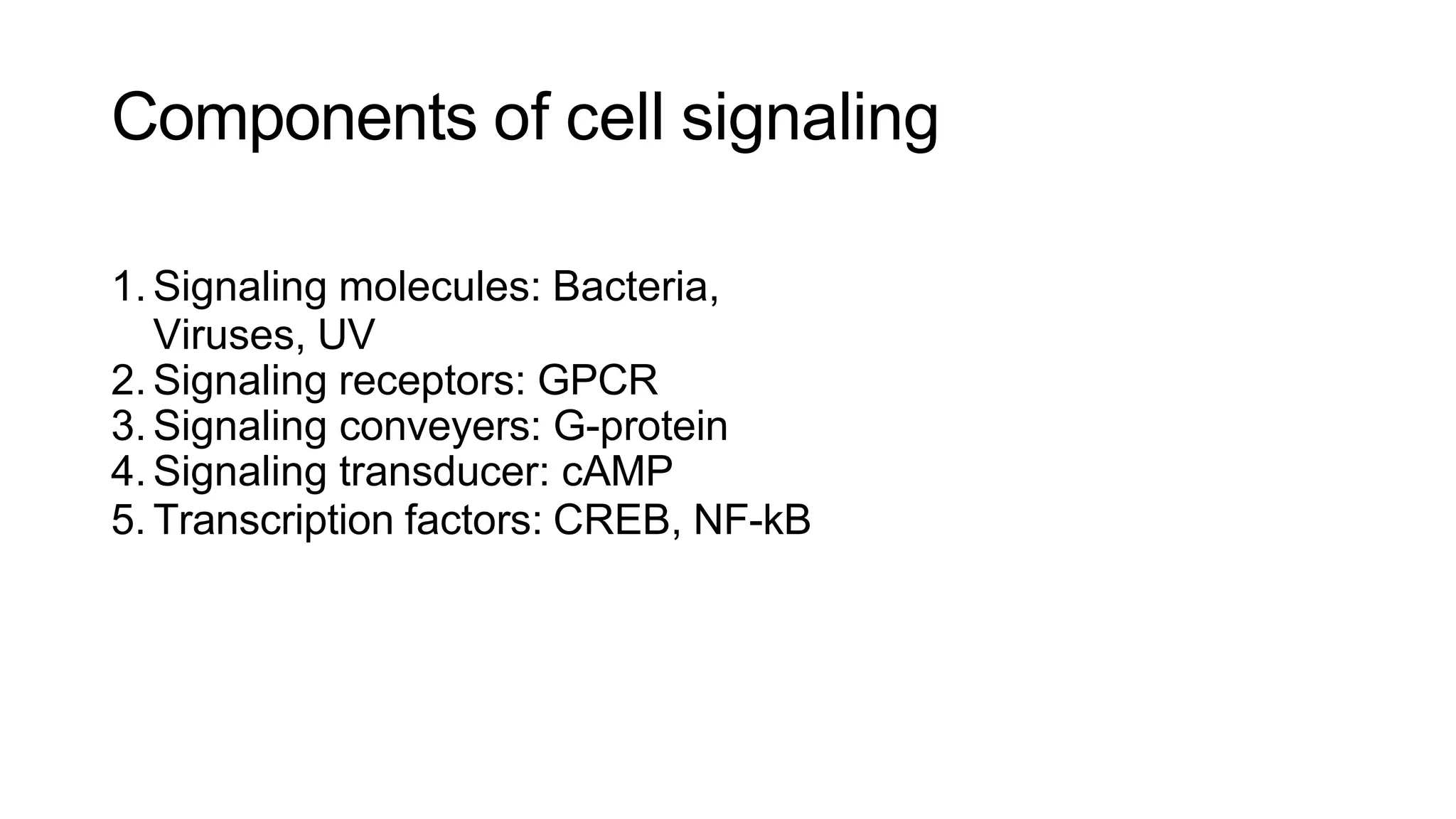
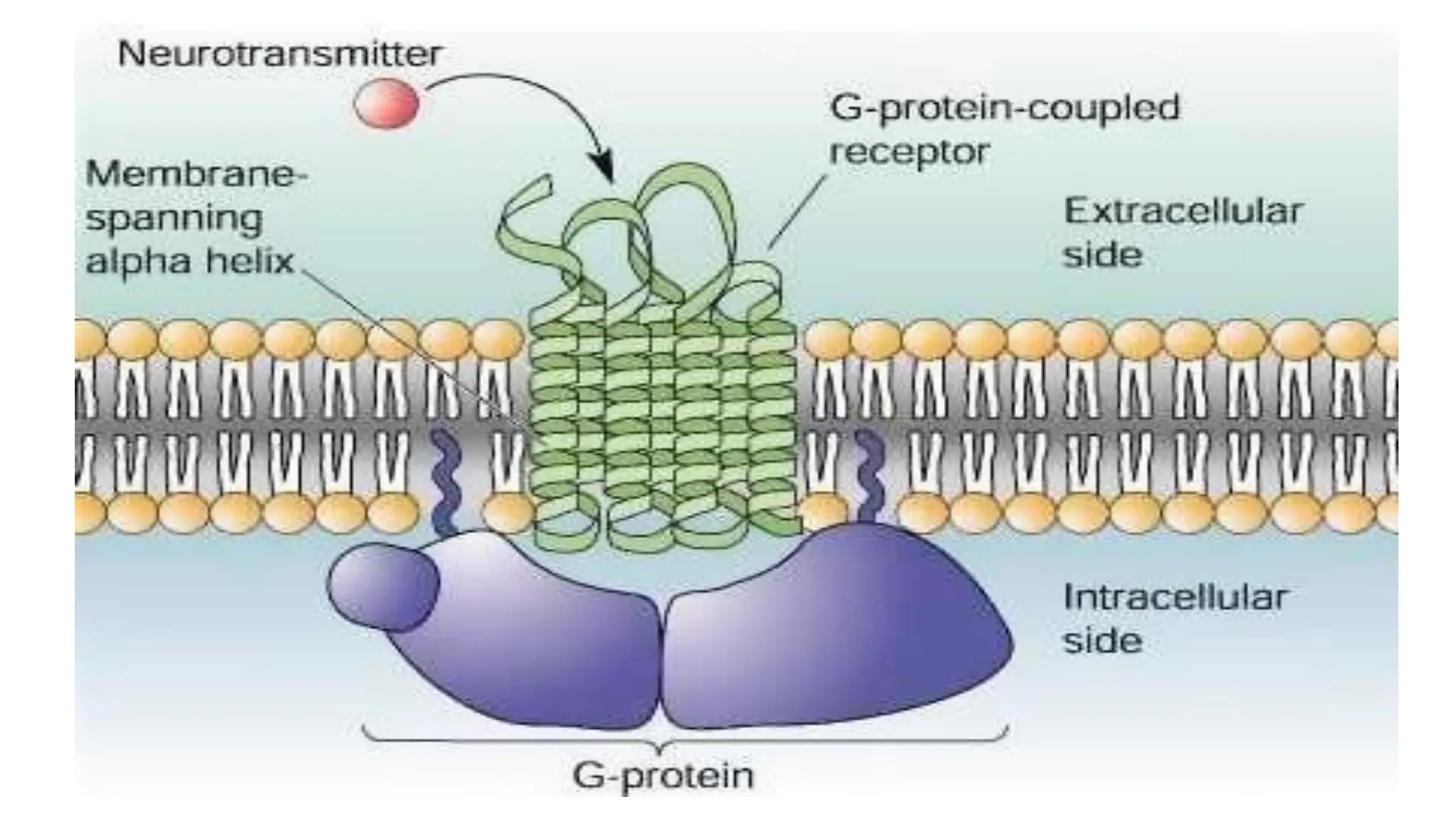
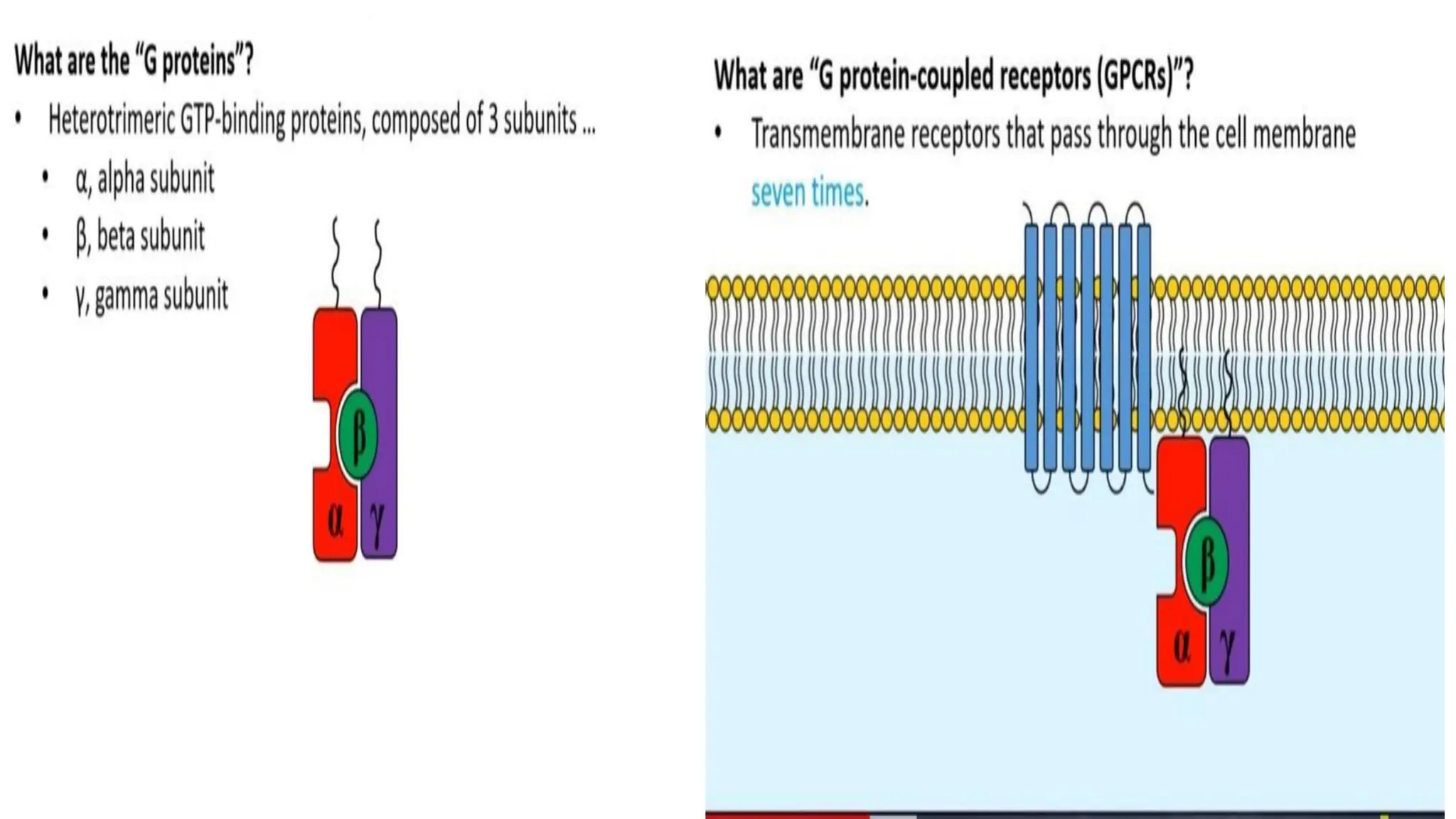
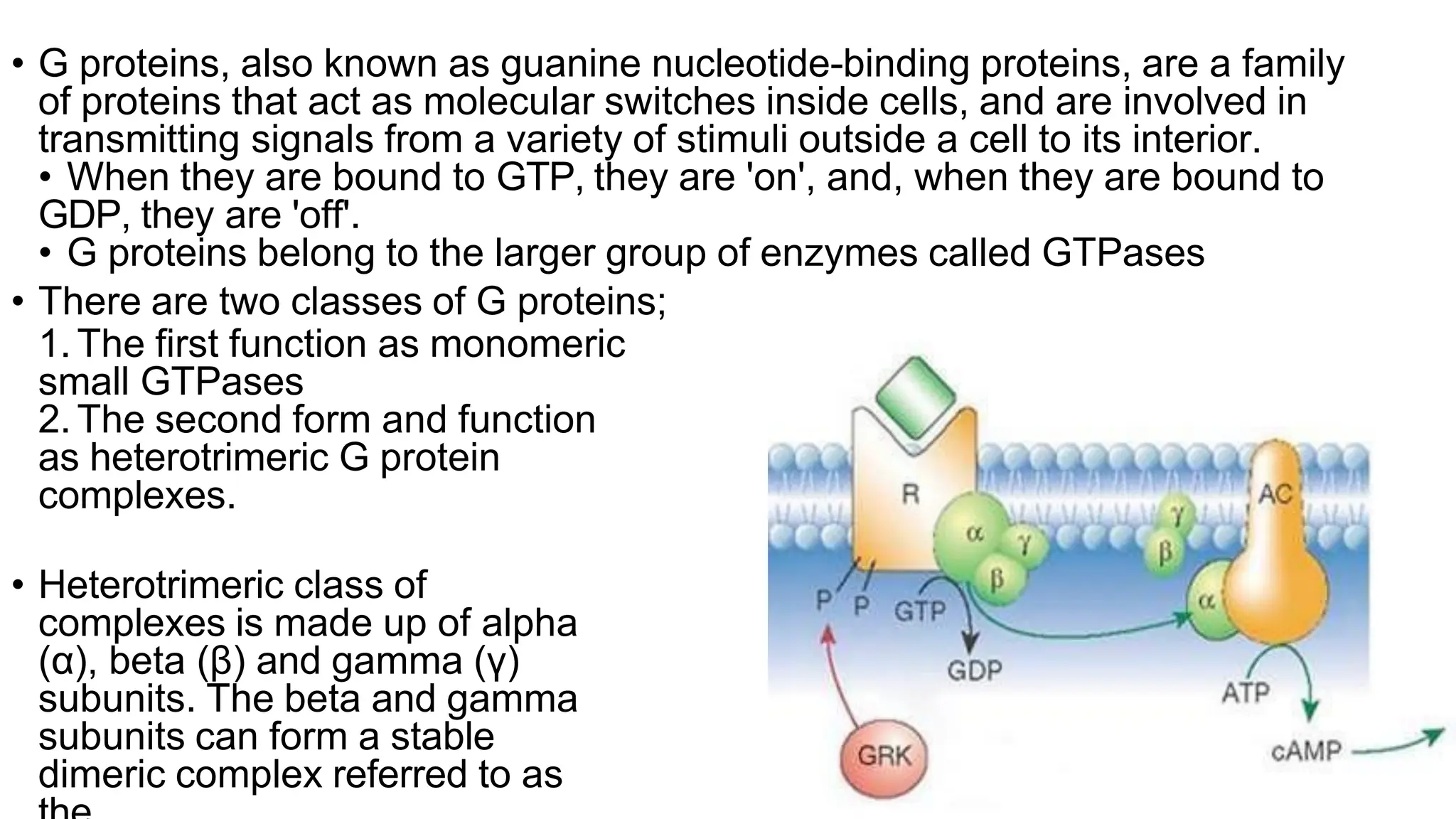
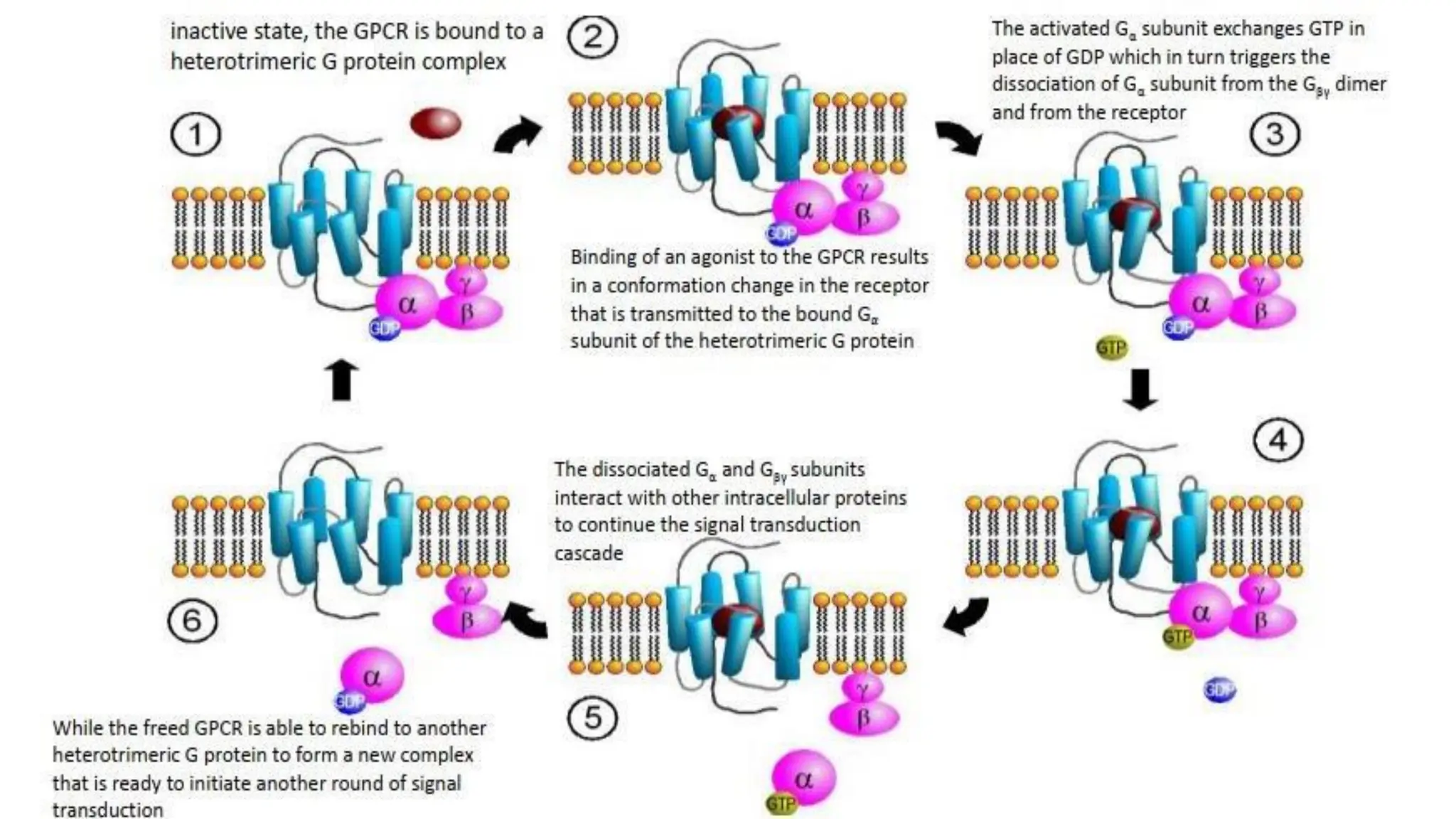
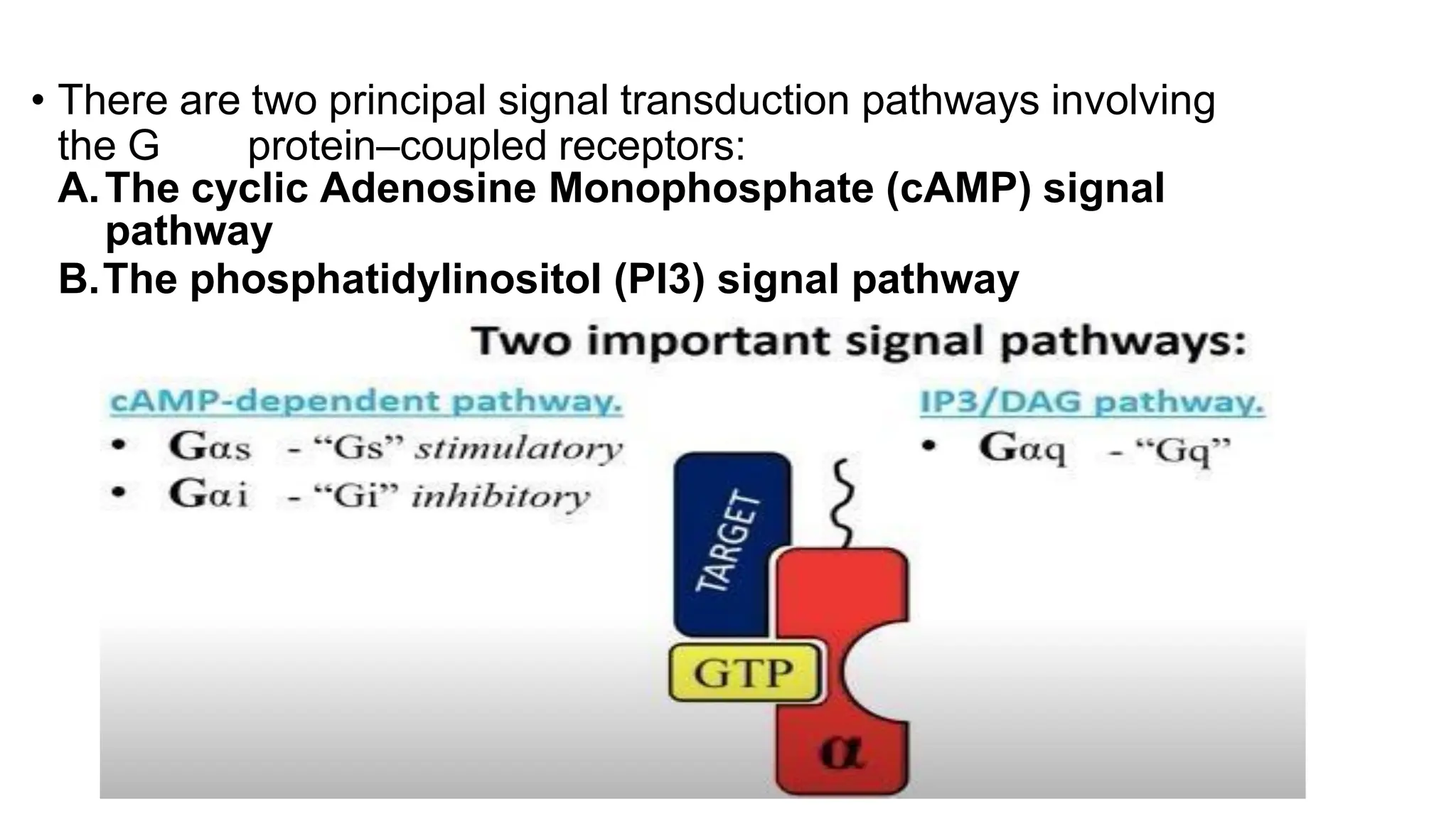
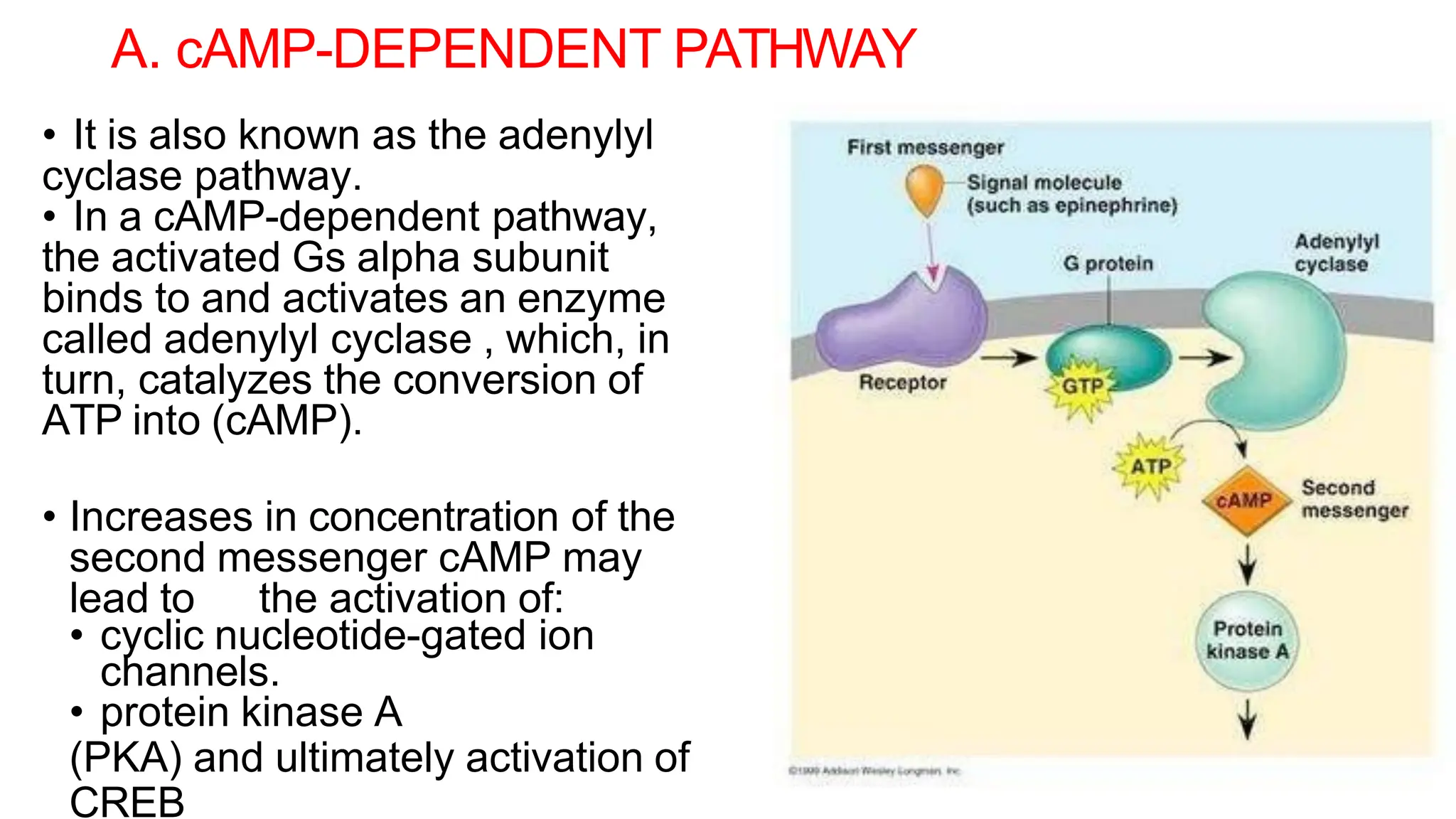
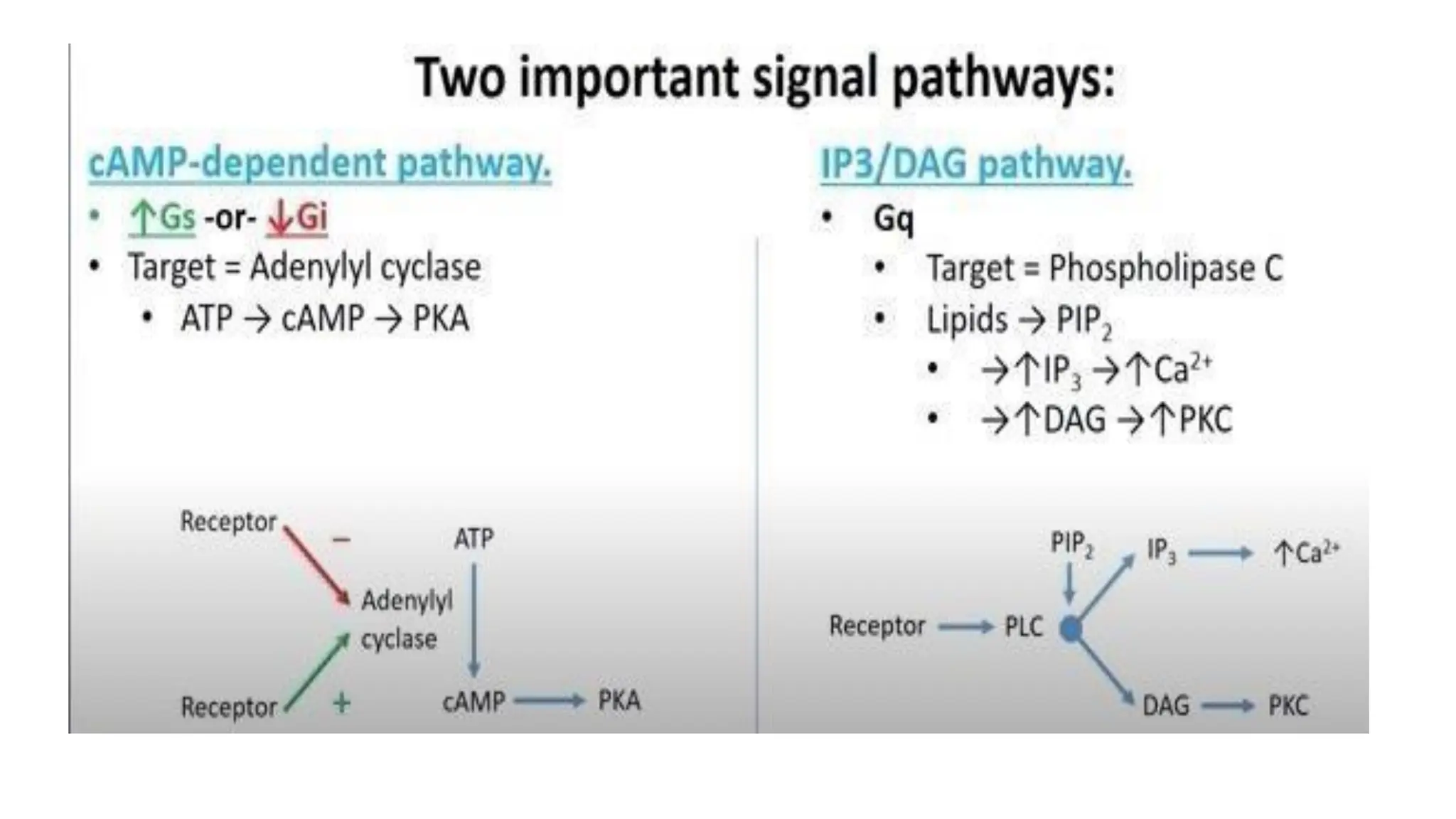
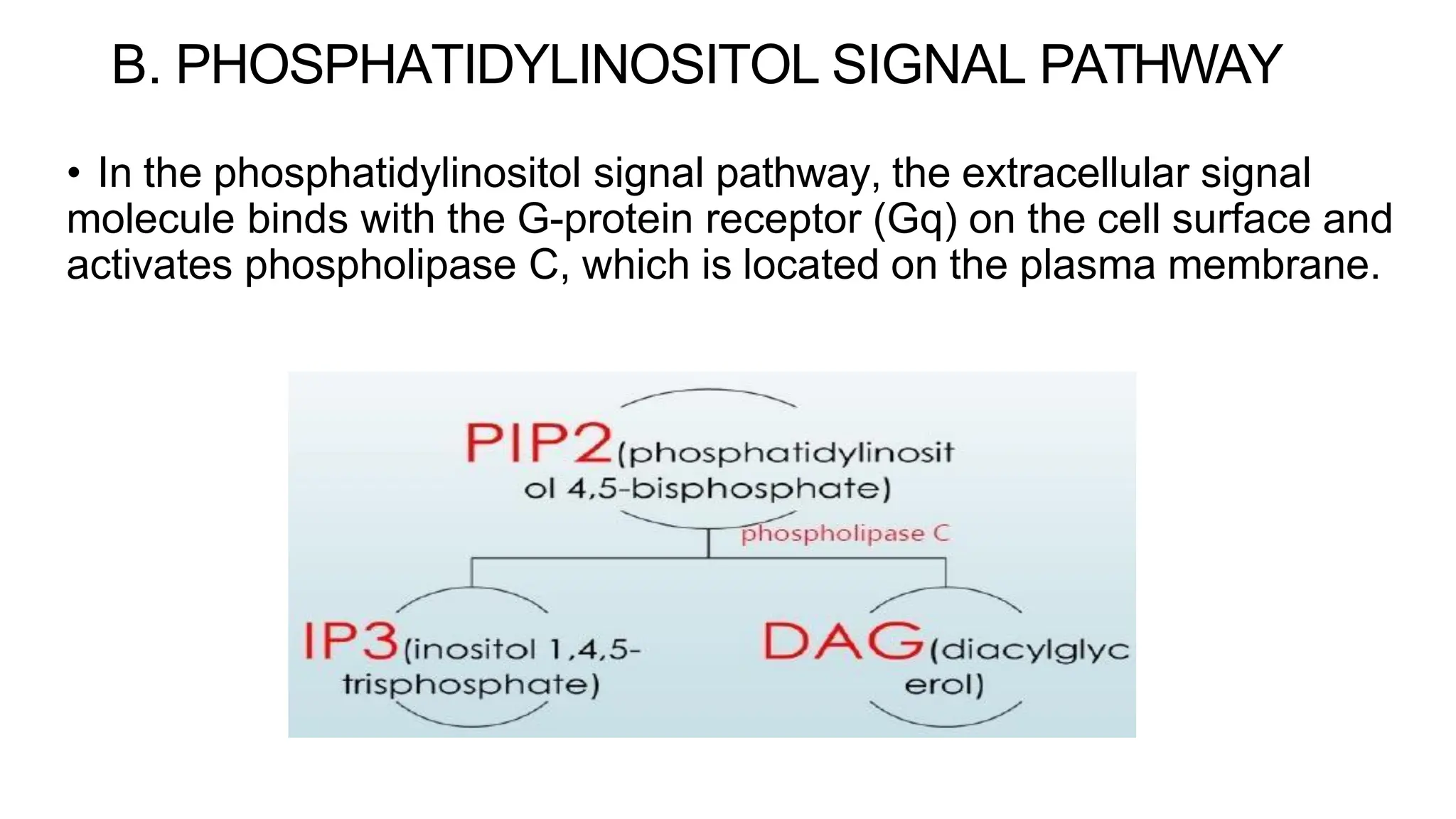
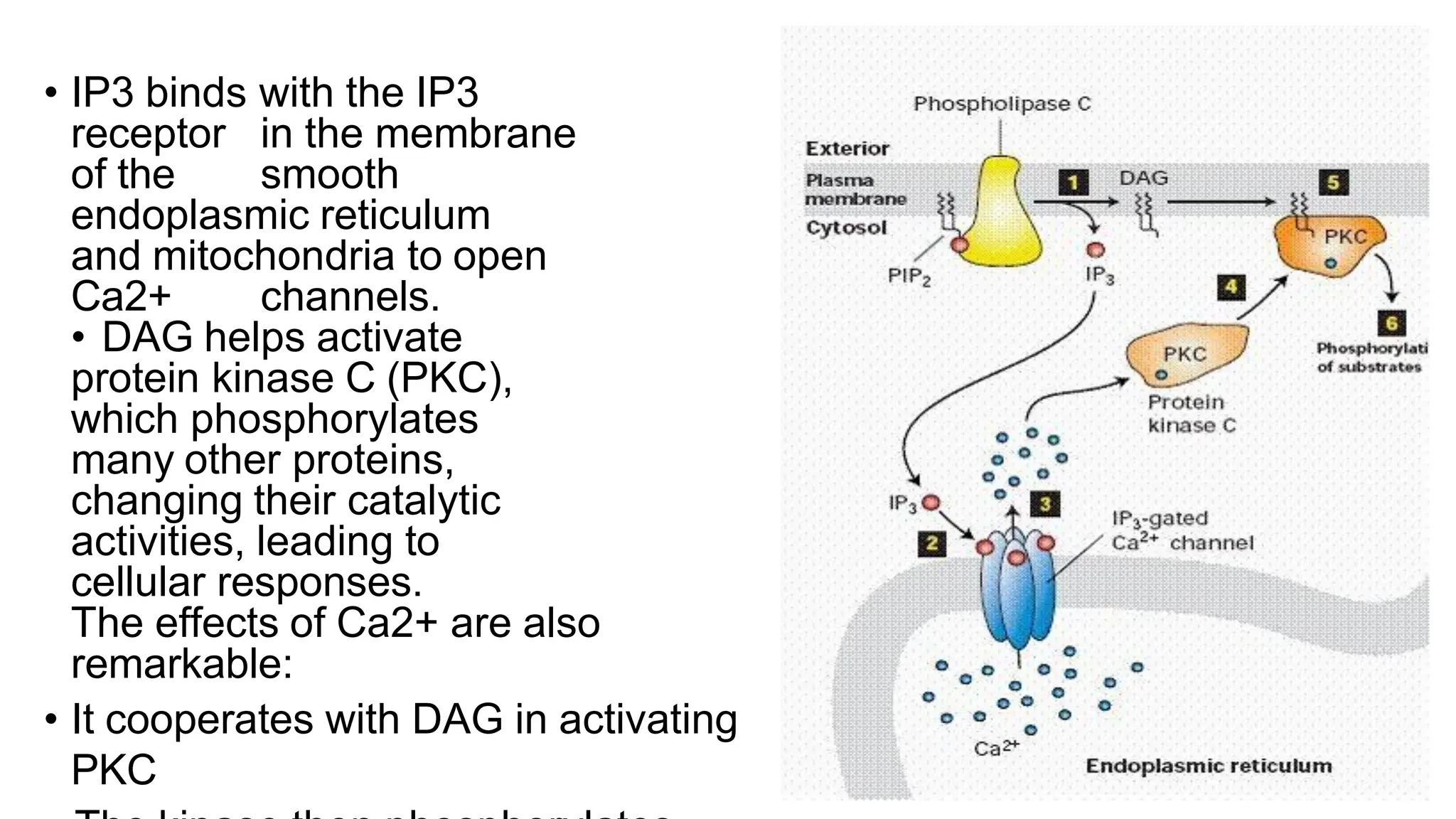
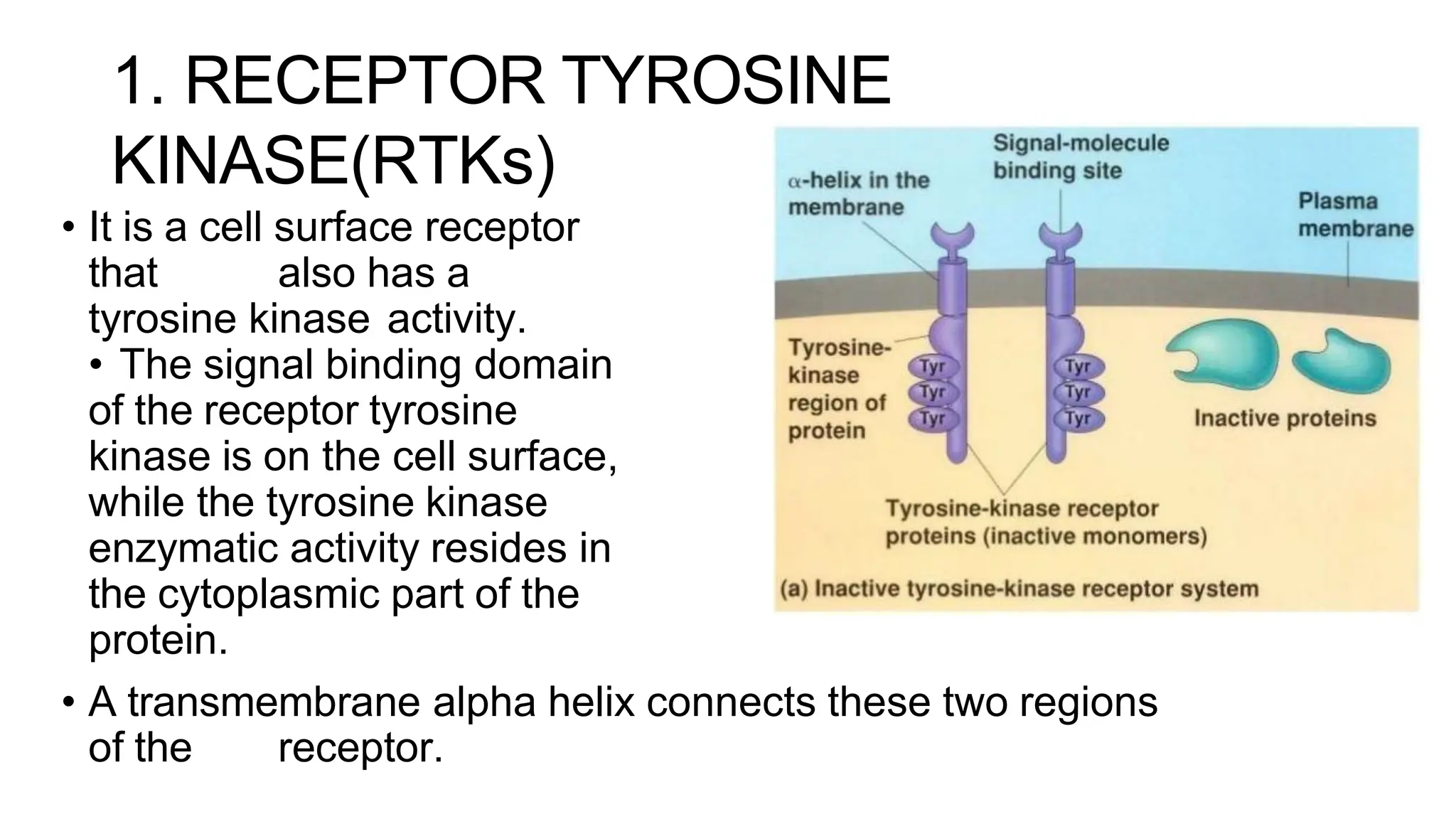
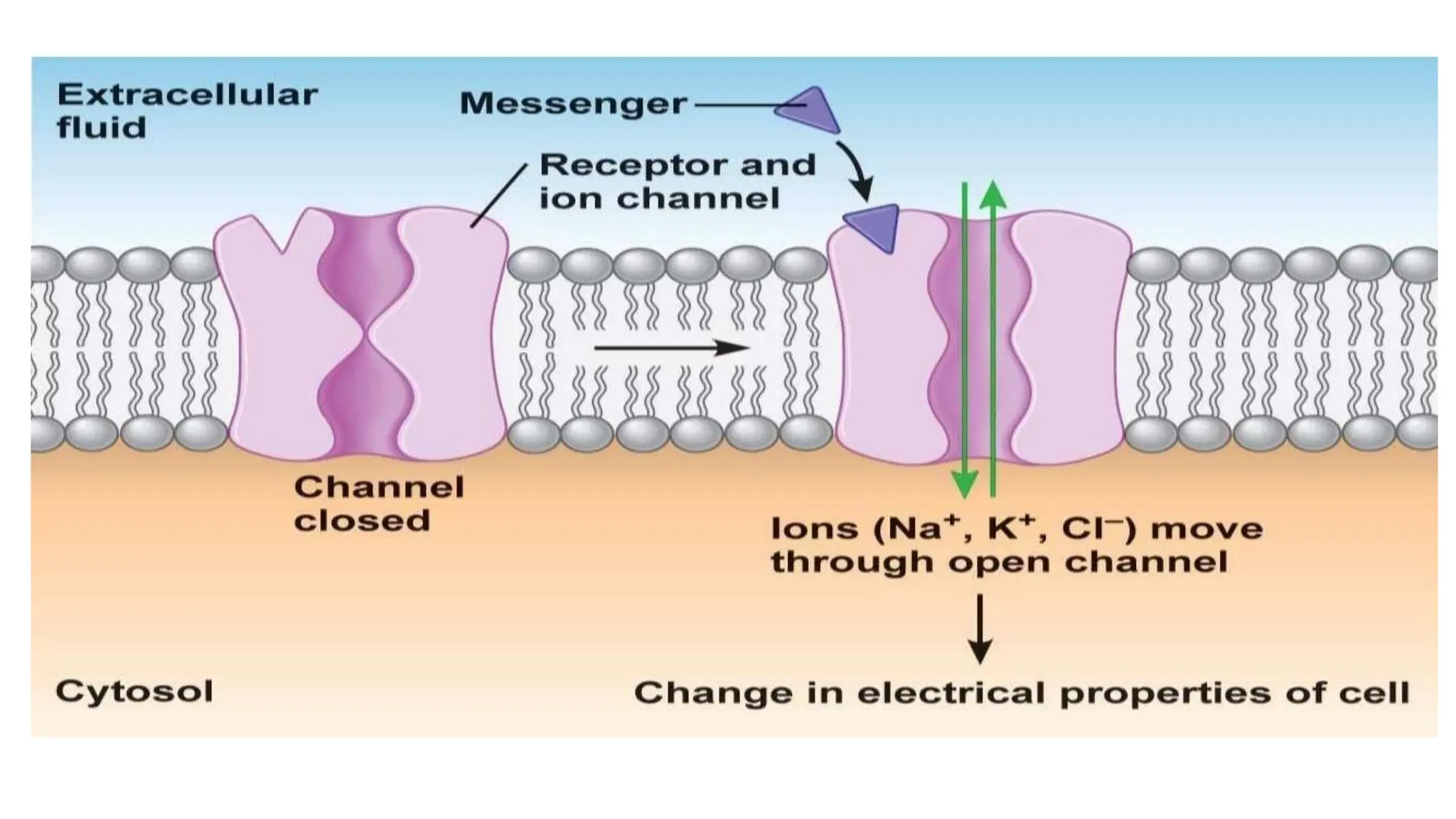
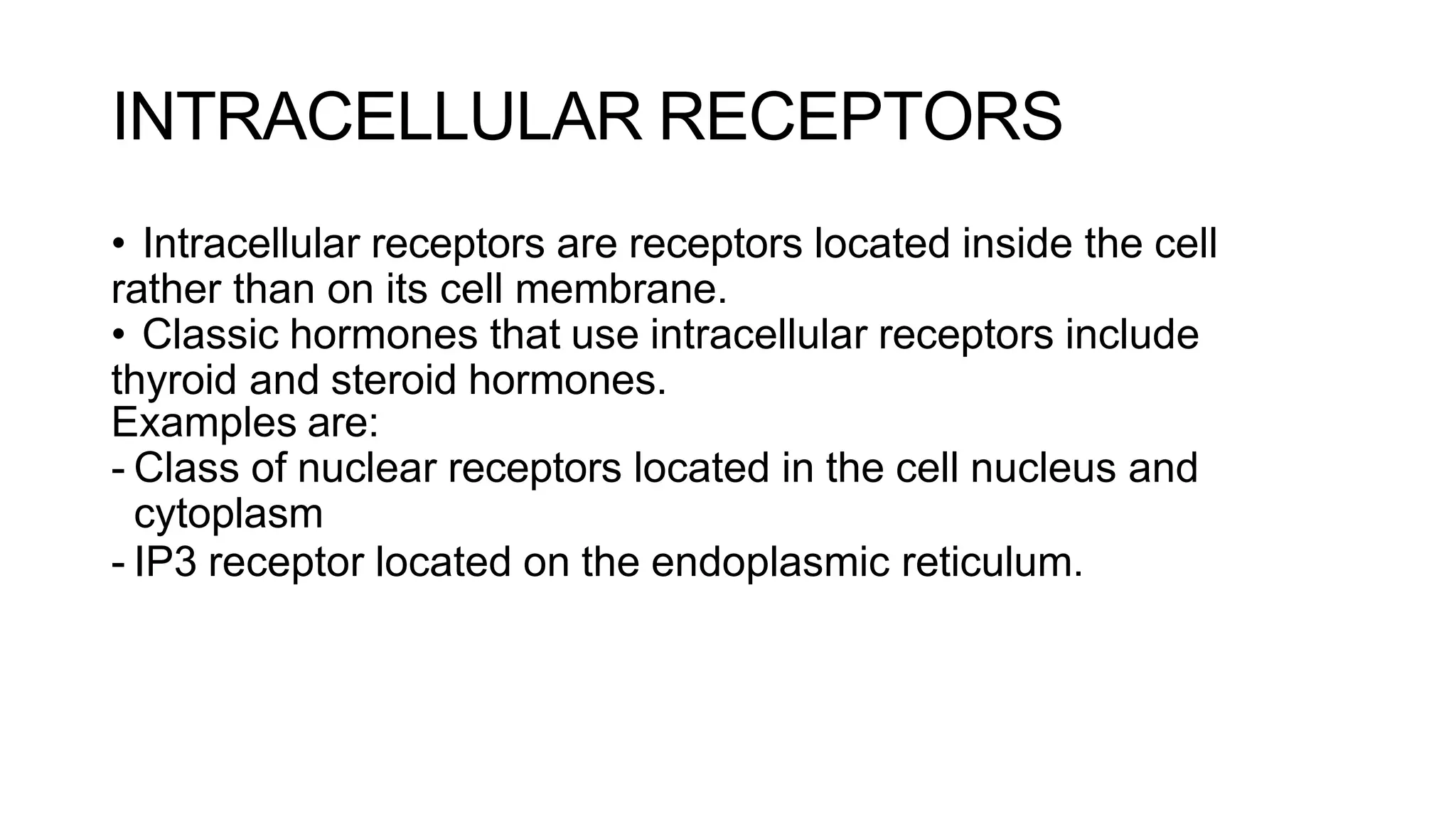
This document discusses cell communication and signal transduction, highlighting their roles in coordinating cellular functions and the implications for diseases like diabetes and cancer. It details the mechanisms of signal transduction, including the roles of different types of receptors (extracellular and intracellular), ligands, and pathways such as G-protein-coupled receptors (GPCRs) and receptor tyrosine kinases. The document emphasizes the importance of effective signal transmission and the biochemical cascades triggered by ligand-receptor interactions in cellular responses.