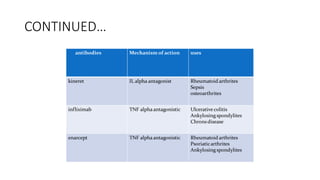
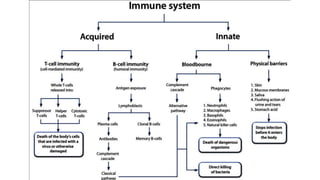
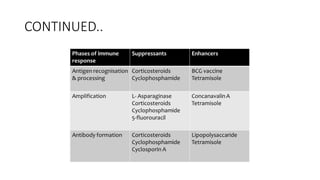
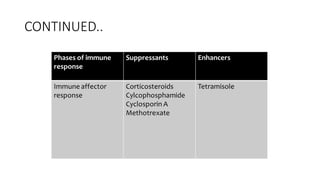
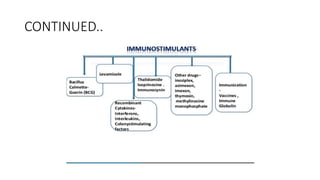
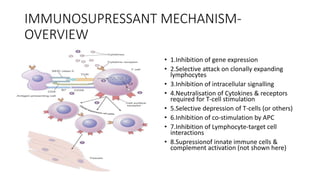
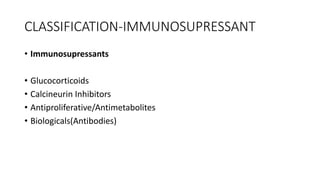
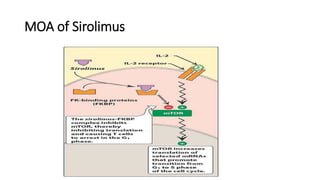
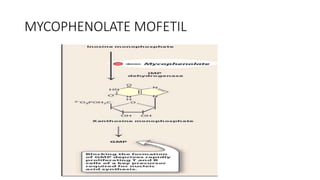
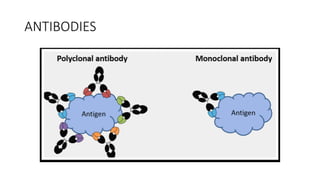
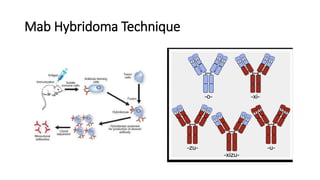
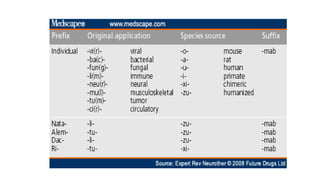
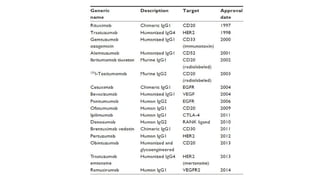
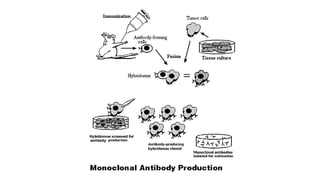
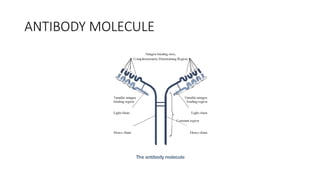
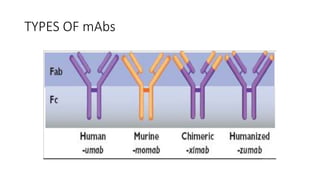
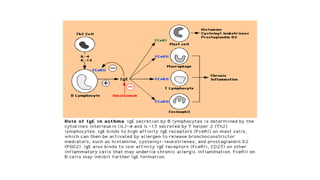
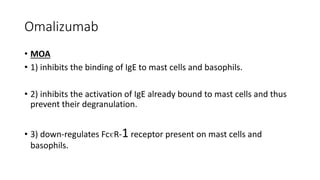
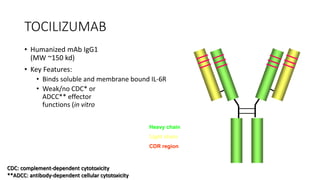
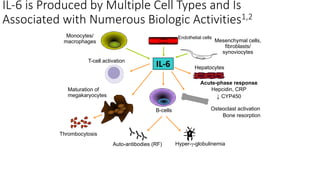
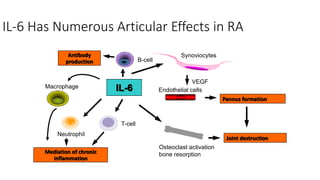
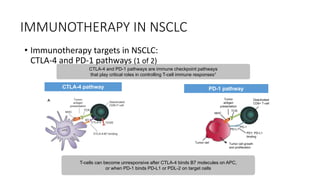
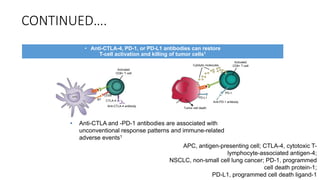
Immunotherapy involves treating diseases by manipulating the immune response, comprising active and passive types. Active immunotherapy aims to stimulate the immune system to attack disease-specific antigens, while passive immunotherapy provides pre-made antibodies to fight disease without triggering an immune response. Various agents, including cancer vaccines, monoclonal antibodies, and immunomodulators, are used for their specific therapeutic effects across multiple conditions.