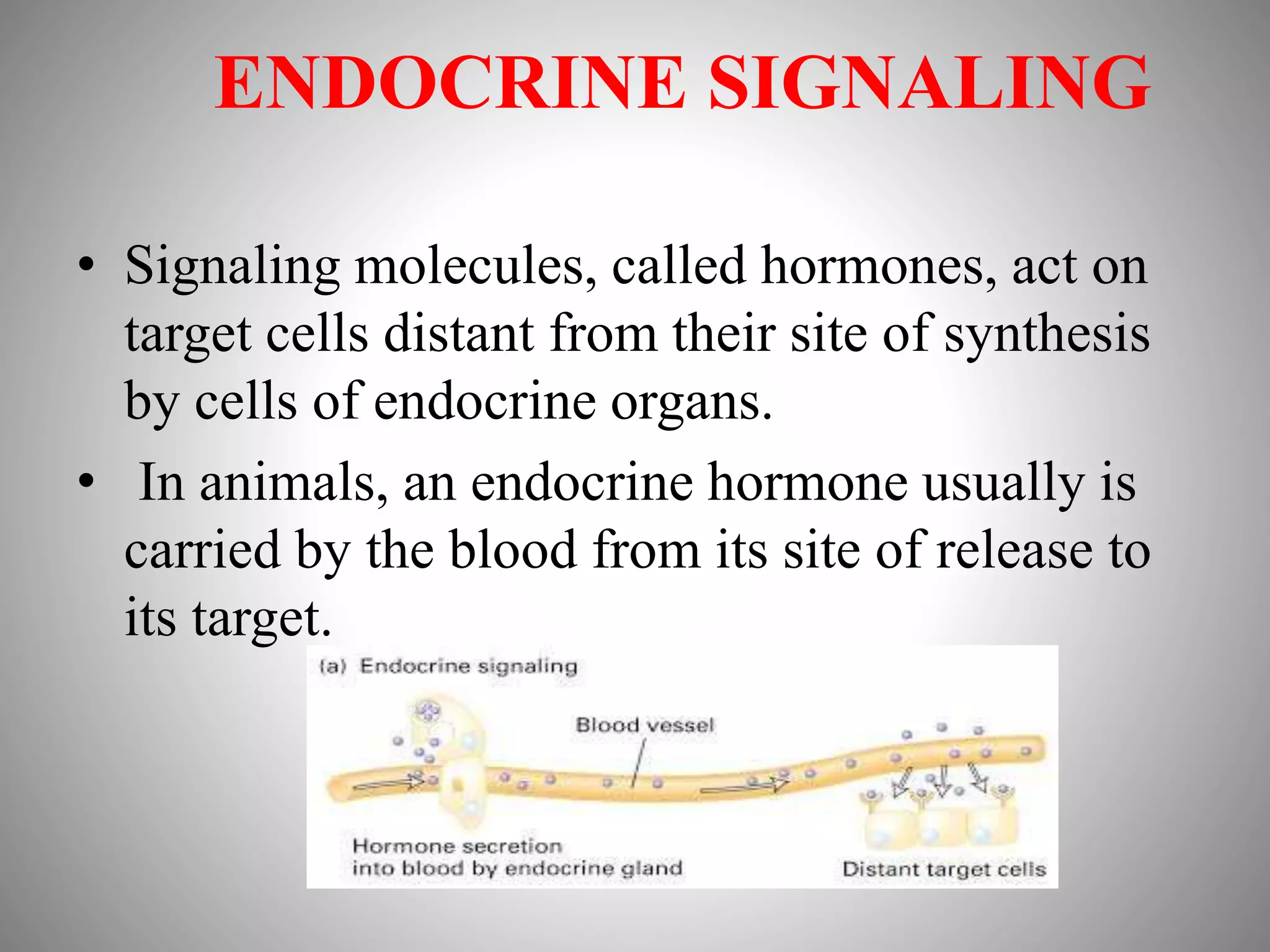
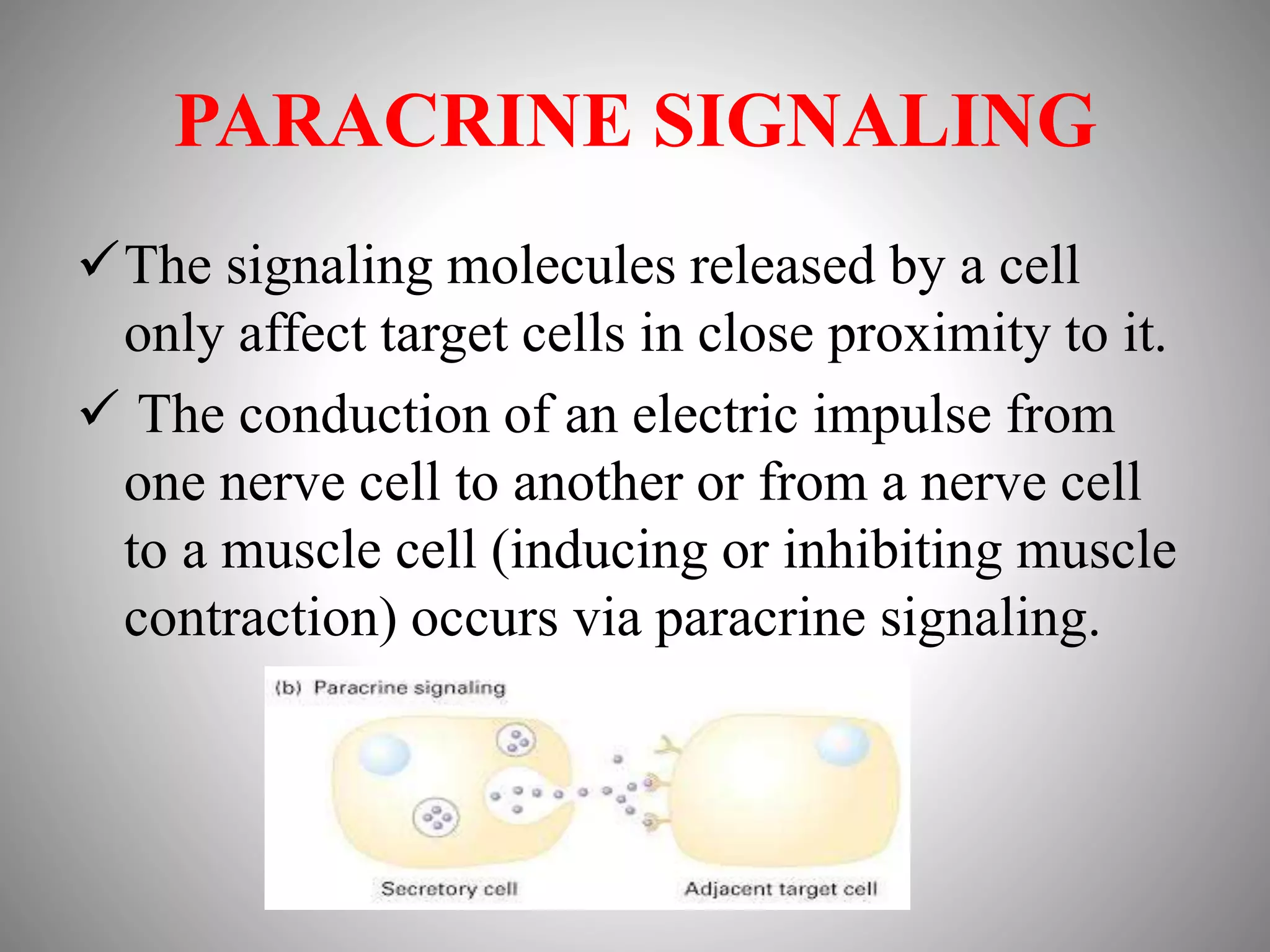
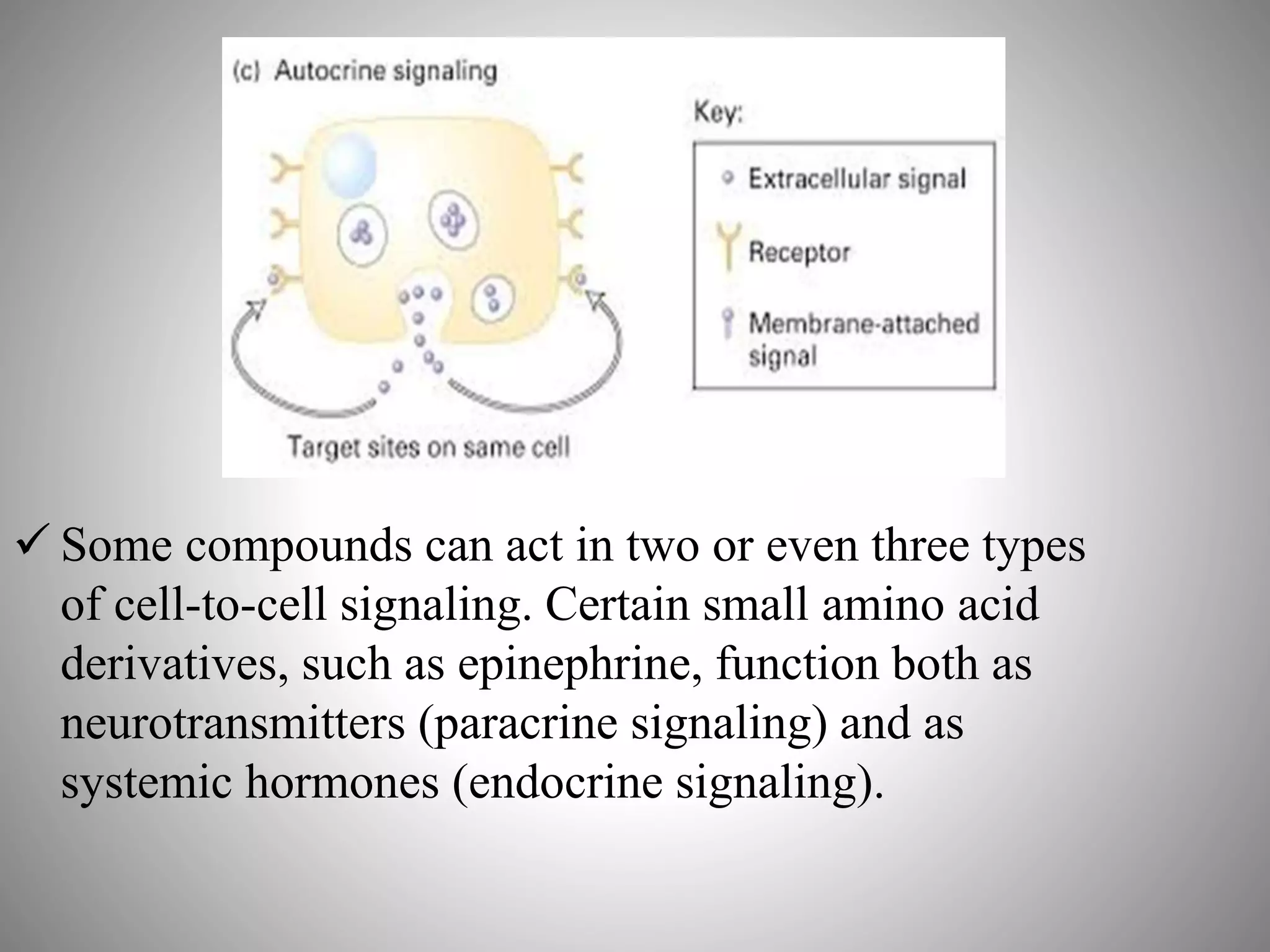
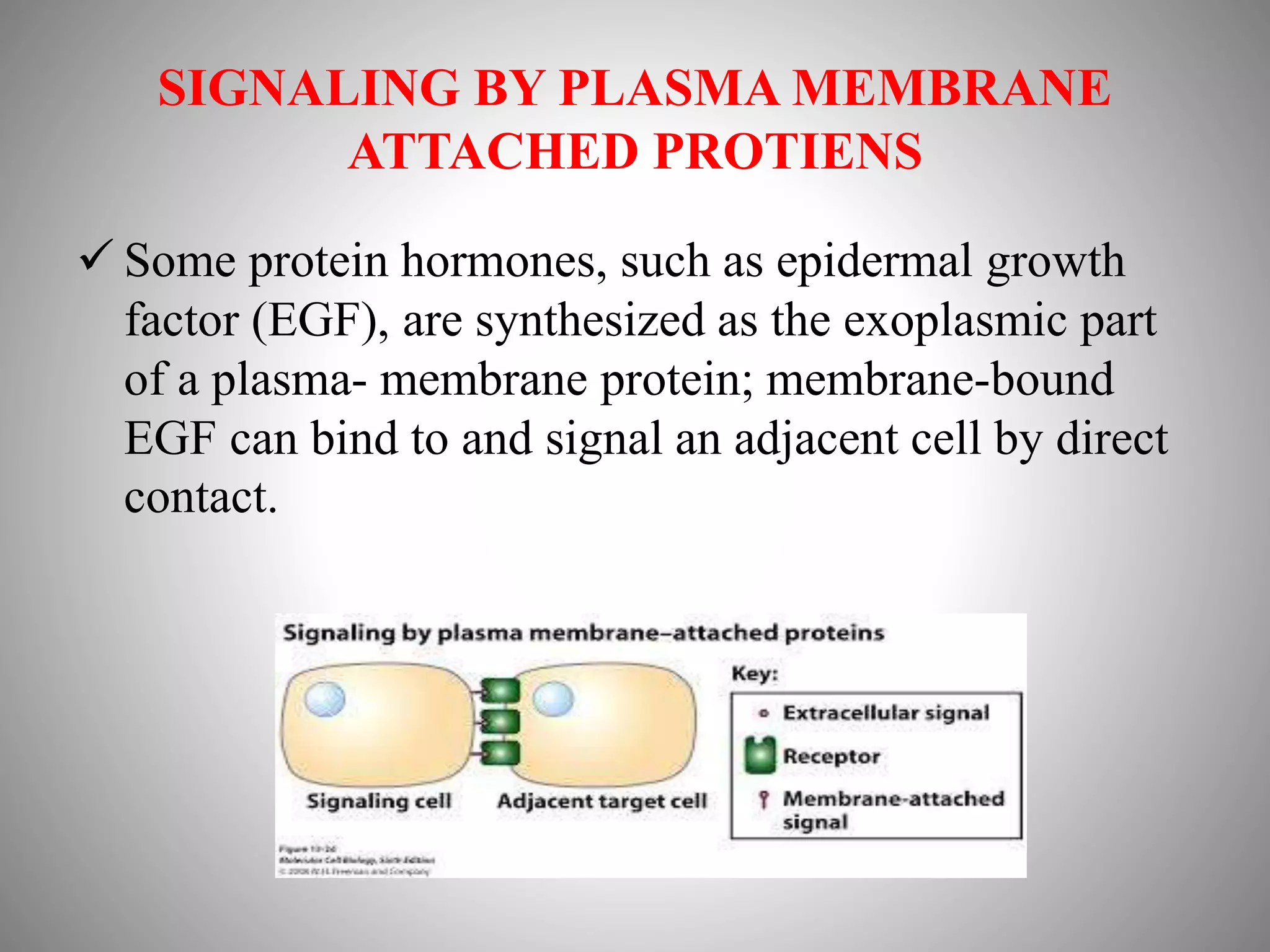
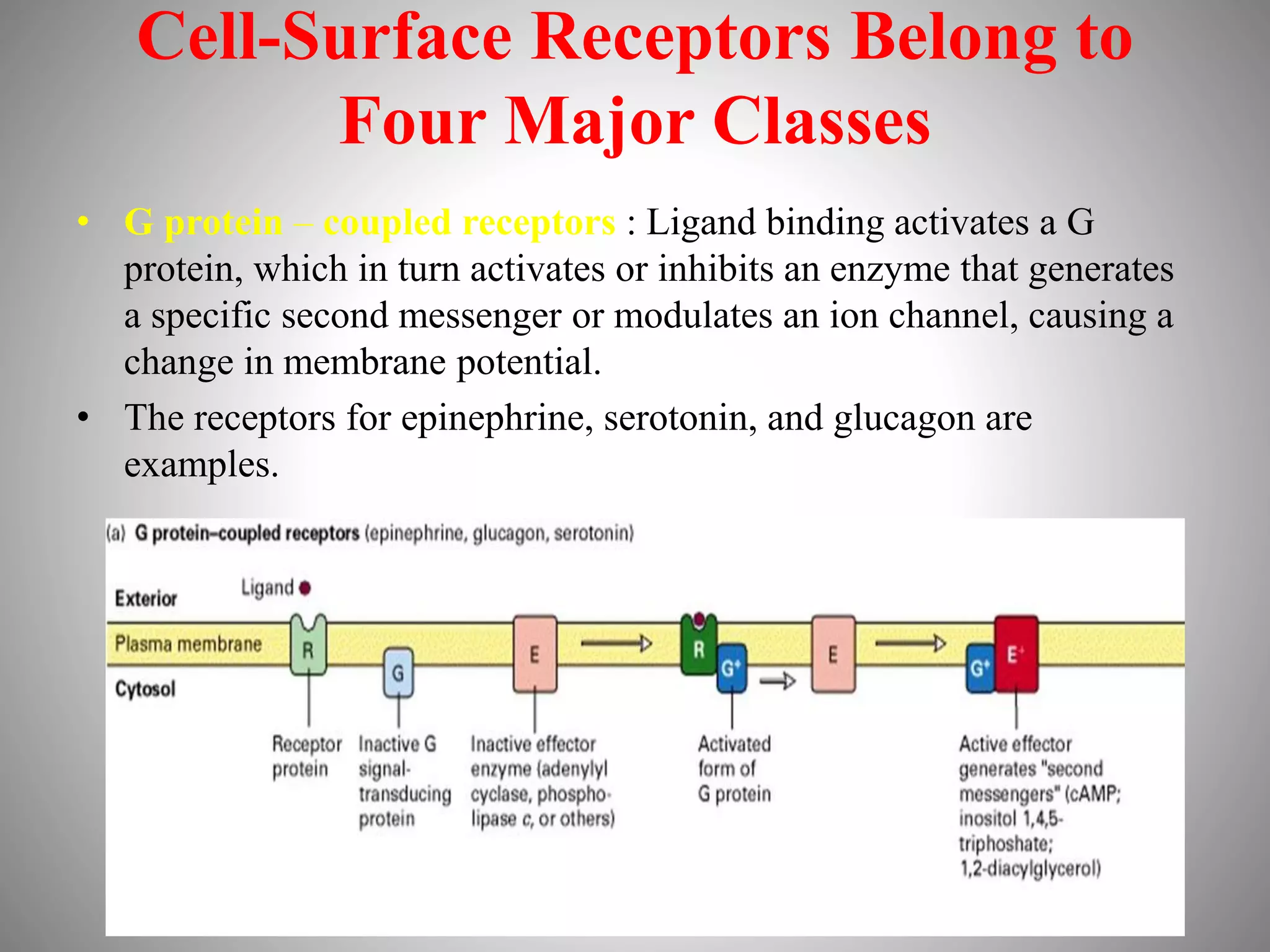
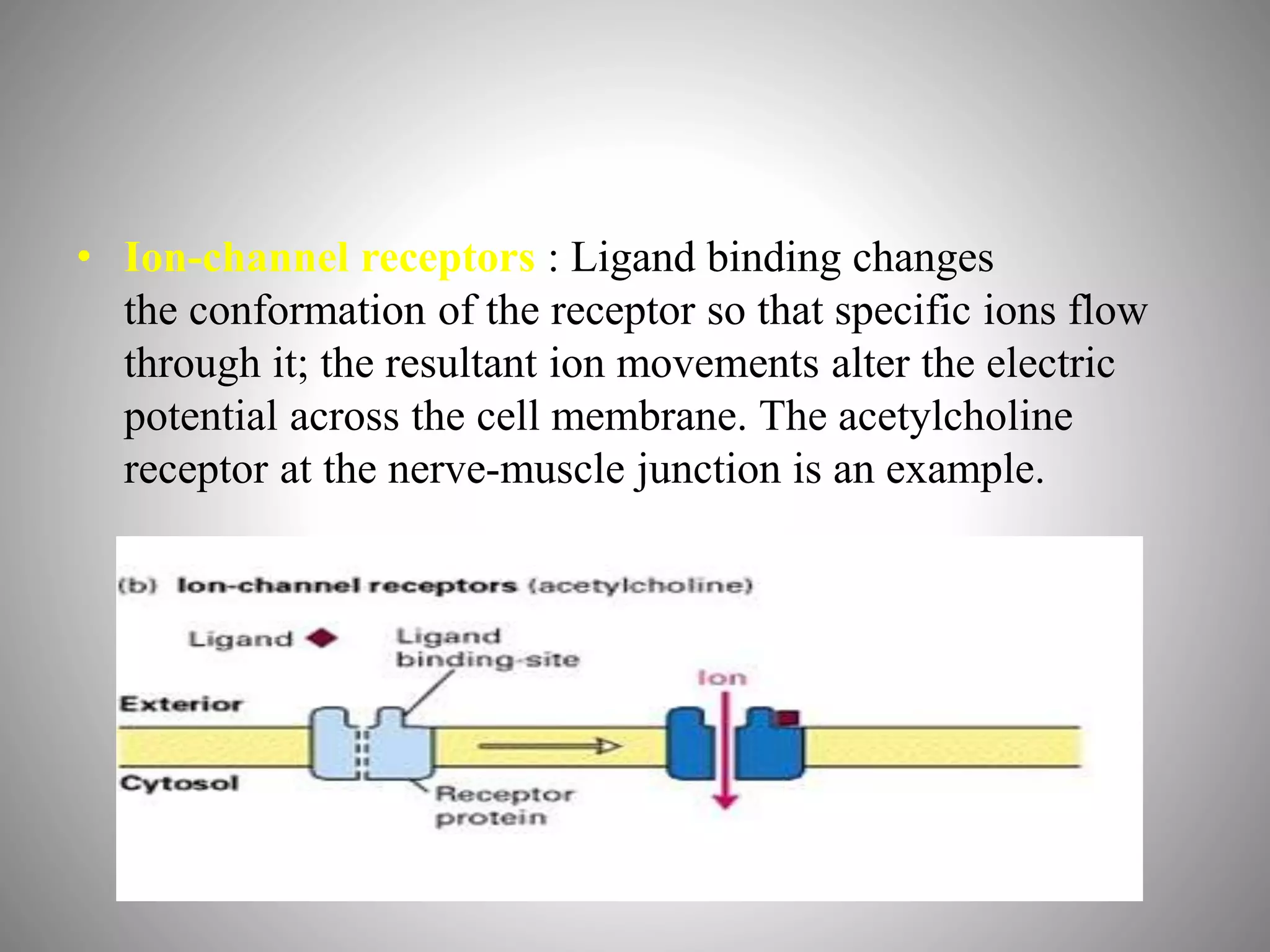
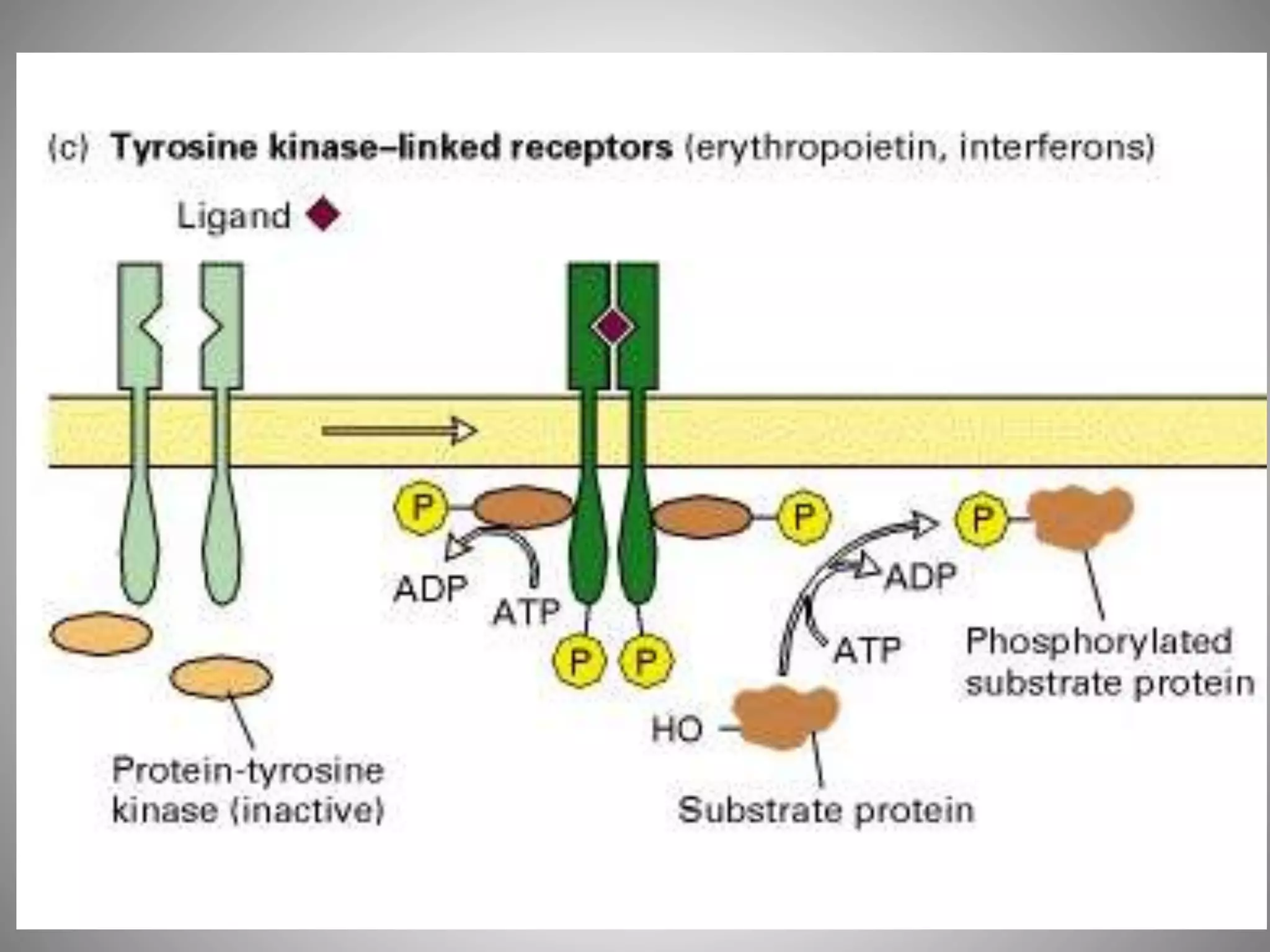
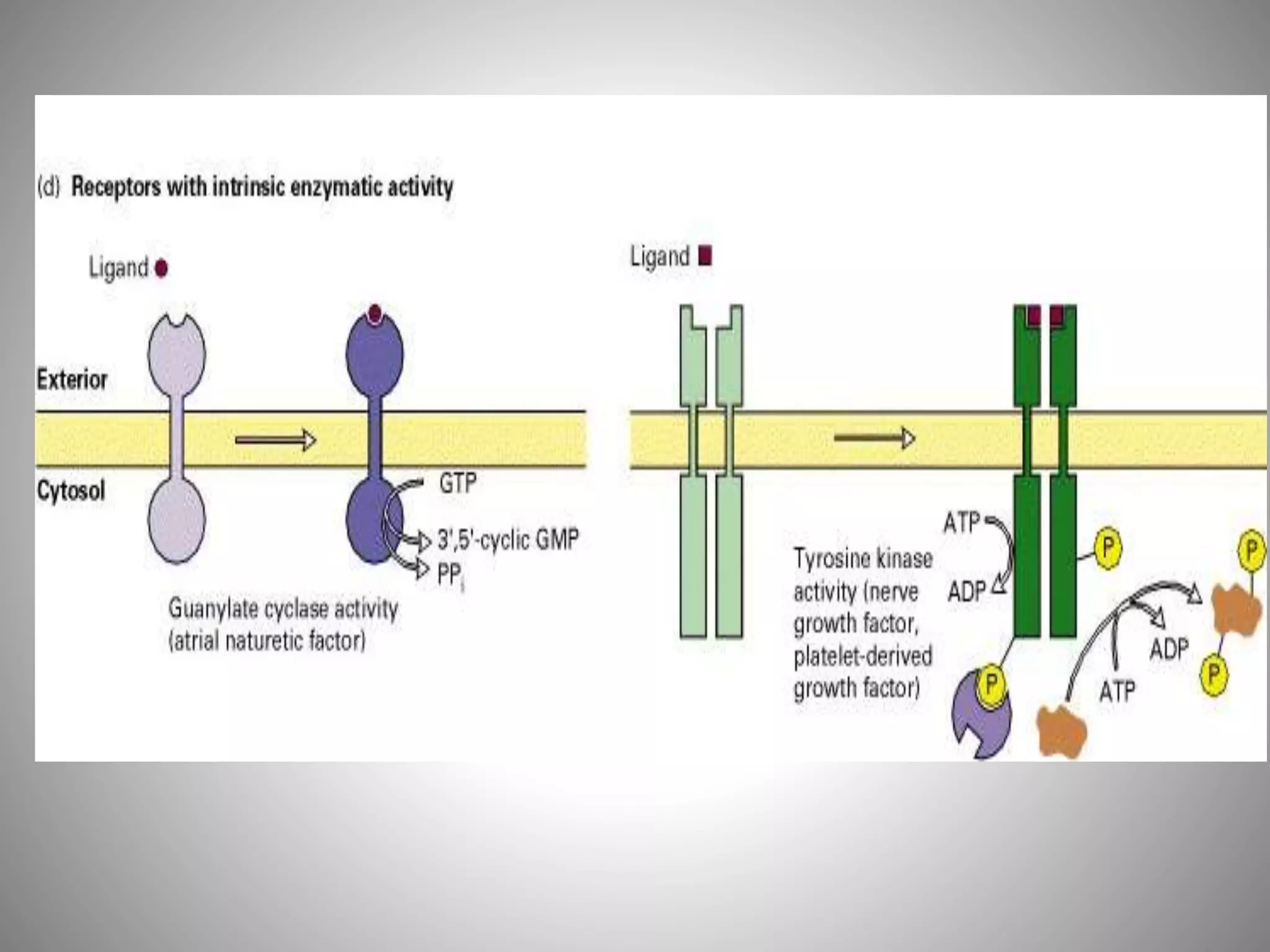
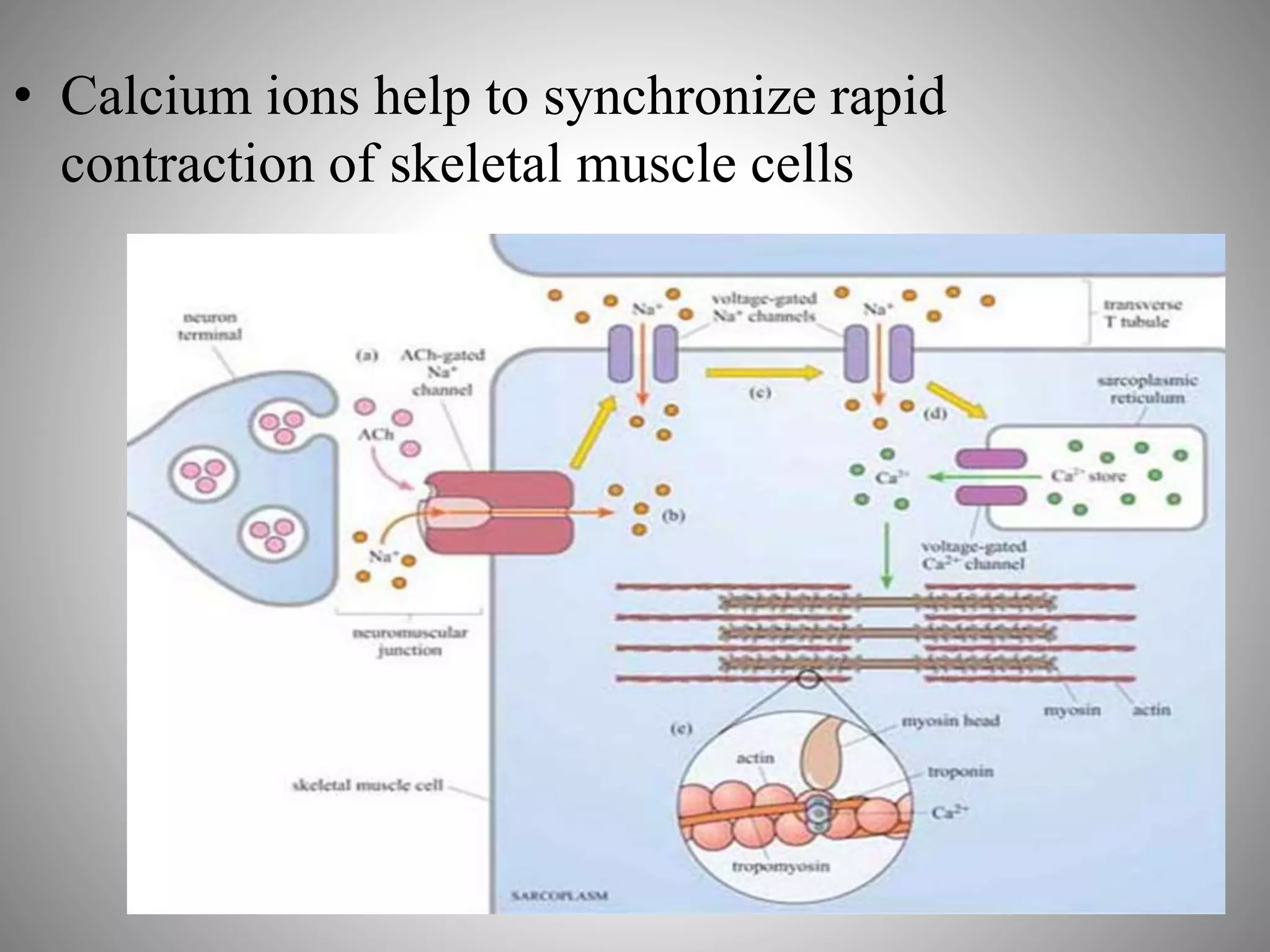
The document discusses extracellular signaling communication involving molecular interactions and regulation through various types of signaling, including endocrine, paracrine, and autocrine signaling. It explains the role of receptors, their properties, and different classes such as G protein-coupled receptors and ion-channel receptors, as well as the mechanisms of signal transduction and the function of second messengers. Additionally, it outlines the physiological responses triggered by ligand-receptor binding and provides an example of acetylcholine's role in muscle contraction.