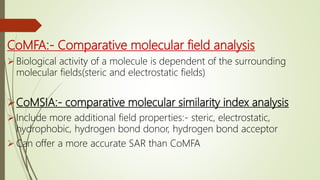
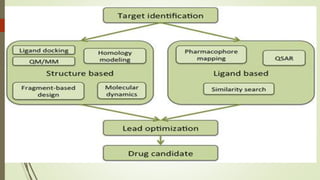
This document discusses alternative methods to animal toxicity testing, including in silico computer simulation methods, in vitro cell culture techniques, using fewer animals, microdosing, and epidemiological and clinical studies. It provides details on regulatory agencies that promote alternative testing methods, as well as specific alternative tests that have been developed and validated according to OECD guidelines to replace or reduce animal use.





![philosophy
Alternative tests are part of philosophy known as the “3’R philosophy “
Replacement, reduction, refinement
Replacement:- refers to the preferred use of non- animal methods over animal methods whenever it is
possible to achieve the same scientific aim.
Reduction:- refers to methods that enable researchers to obtain more information from the same number of
animals.
Refinement:- refers to methods that minimize potential pain, suffering, or distress, and enhance animal welfare
for the animals used in experiment.
Research initiatives and legislation
Research initiatives implemented to achieve 3R principle in research institutes and colleges
SEURAT-1[EU] USDA[US]
EUROECOTOX[EU] AWIC[CAD]
AXLR8[EU]](https://image.slidesharecdn.com/alternativemethodstoanimaltoxicitytesting-201117090224/85/Alternative-methods-to-animal-toxicity-testing-6-320.jpg)