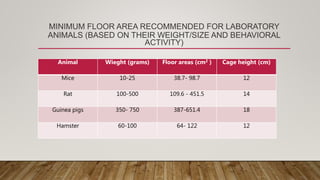
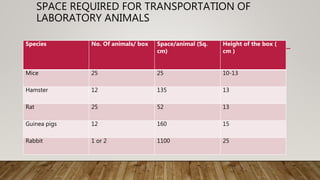
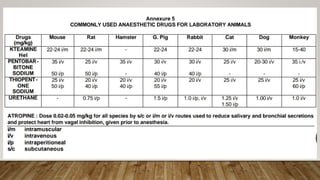
This document summarizes the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines for animal experimentation. It discusses the goals of humane animal care and avoiding unnecessary pain. It provides recommendations for housing, veterinary care, disease control, and husbandry practices. These include maintaining hygienic facilities, providing social environments, and disposing of waste properly. The document also outlines functions of the CPCSEA like registering facilities, approving protocols, and enforcing compliance. Standard operating procedures, transportation, anesthesia, and euthanasia methods are also addressed to ensure ethical treatment of laboratory animals.