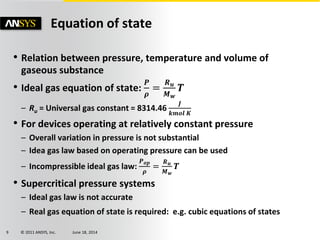
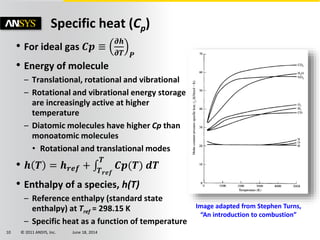
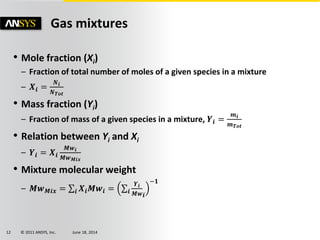
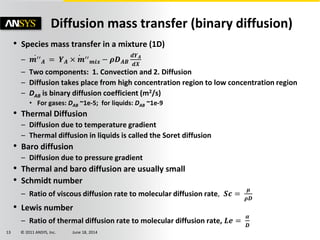
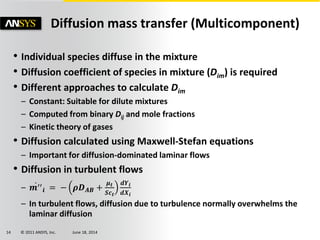
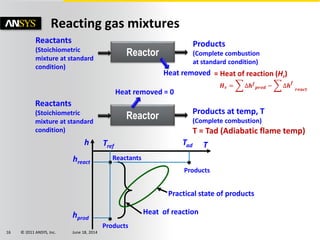
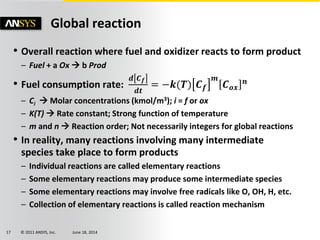
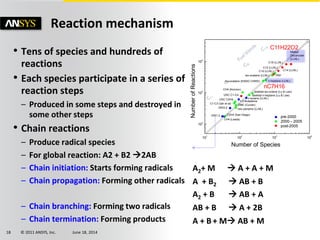
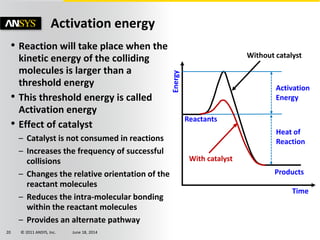
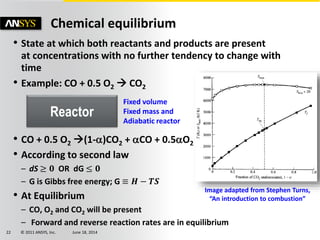
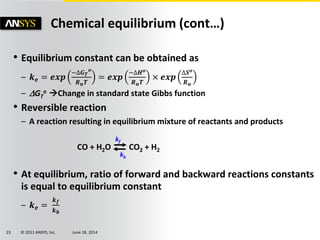
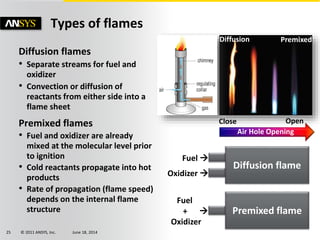
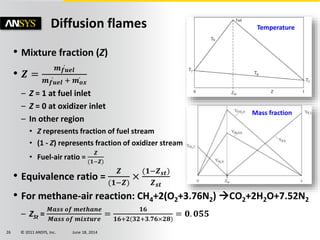
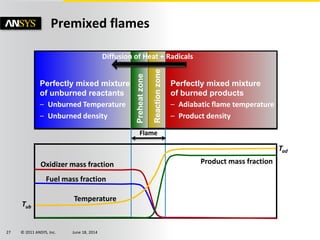
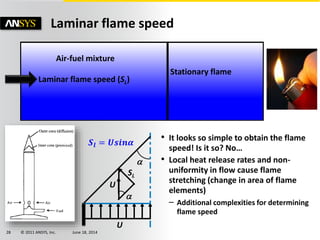
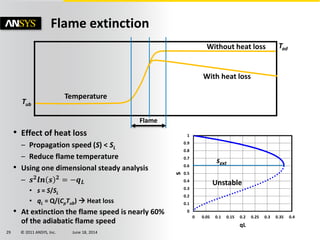
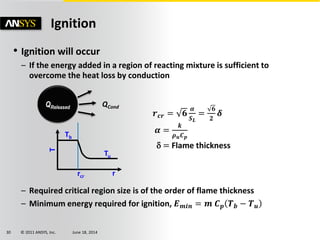
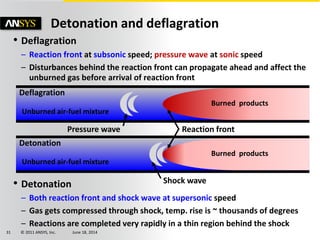
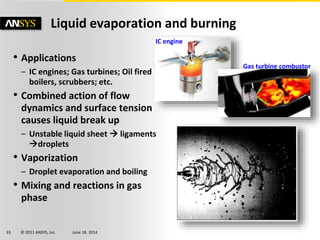
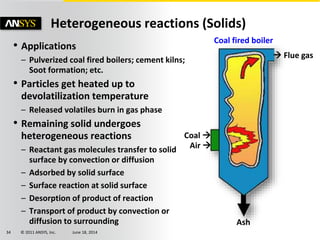
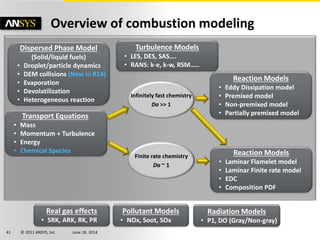
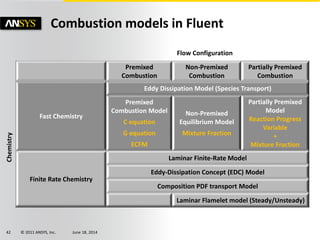
The document is a technical presentation on combustion simulation, detailing fundamental concepts and various modeling techniques within ANSYS CFD. It explores reacting flows, combustion chemistry, pollutant modeling, and heterogeneous reactions involving solid and liquid fuels. The content covers definitions, principles of gas mixtures, types of flames, and the dynamics of chemical reactions relevant to combustion processes.