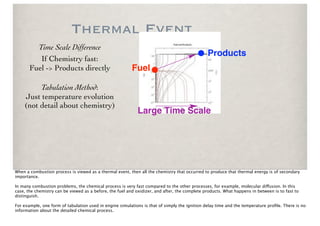
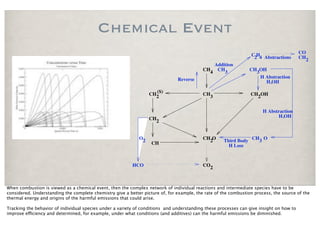
The document discusses the importance of chemical kinetics in modeling combustion processes, emphasizing the need for both detailed chemical and simplified thermal models depending on the complexity of the conditions. It highlights how understanding combustion is vital for improving energy efficiency and reducing emissions, which is essential in a society focused on clean energy. The balance between computational detail and model accuracy is crucial for effective combustion modeling and optimizing the combustion process.