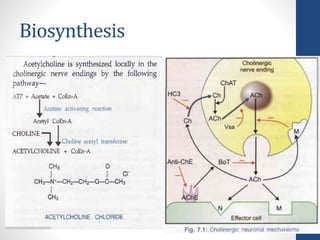
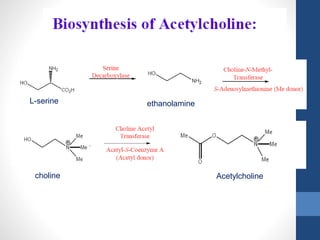
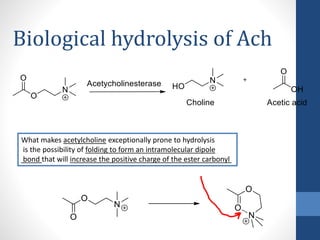
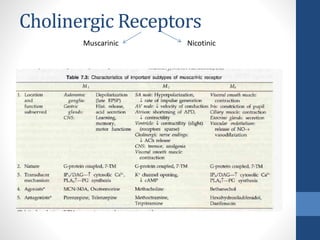
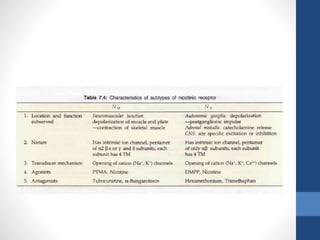
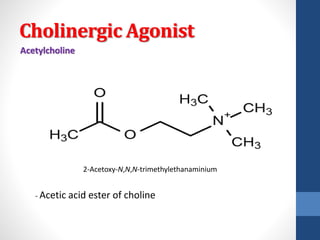
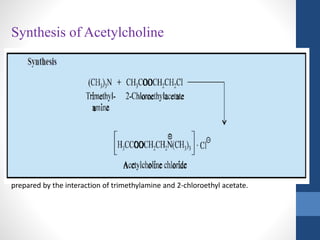
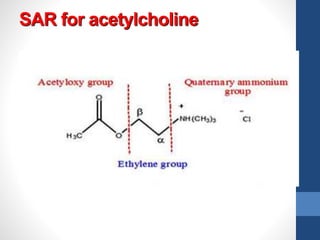
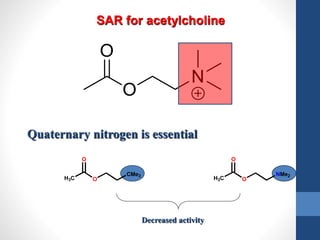
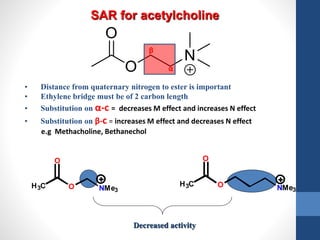
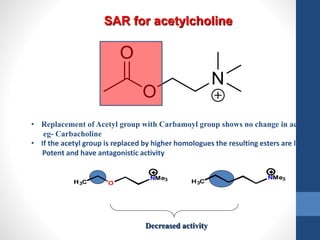
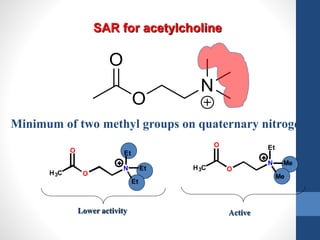
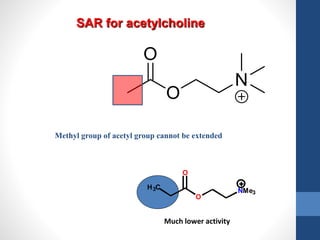
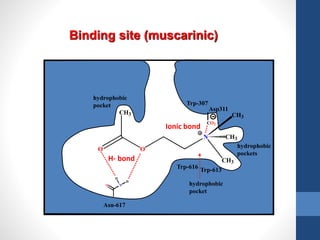
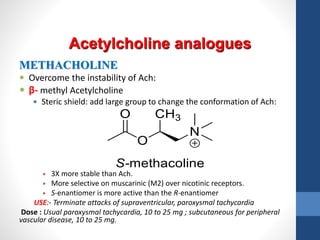
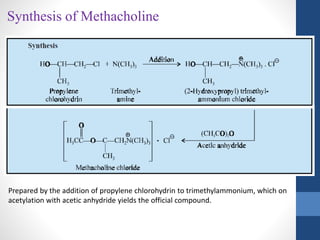
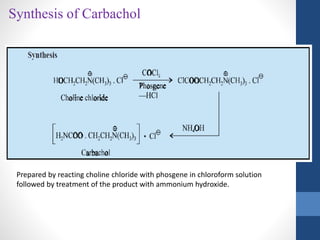
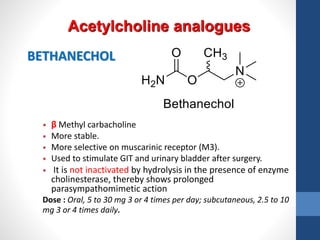
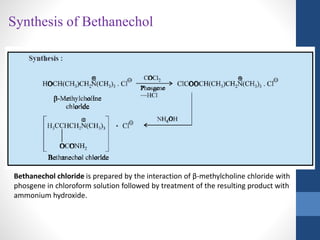
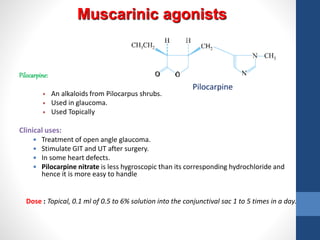
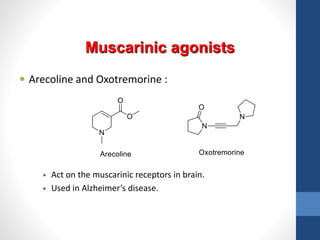
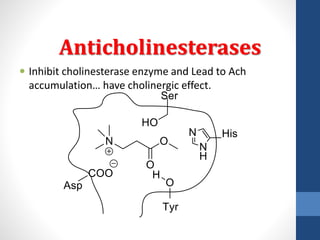
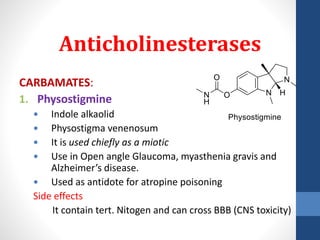
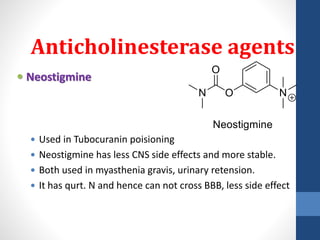
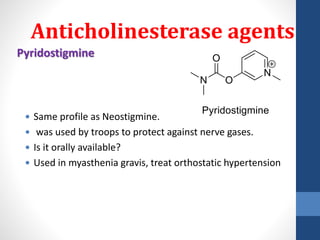
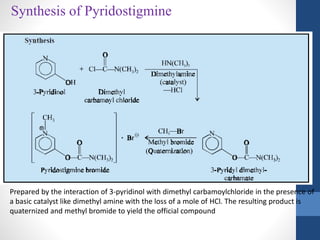
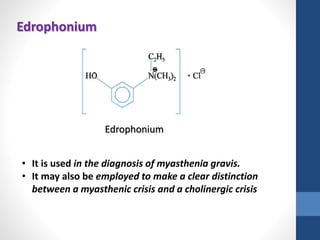
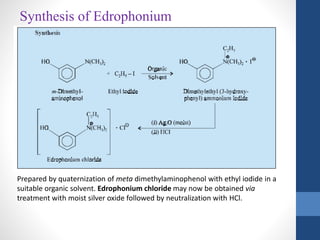
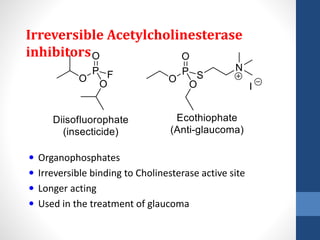
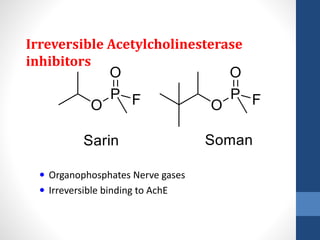
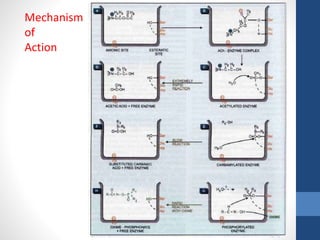
This document discusses cholinergic agents, which are drugs that produce effects similar to acetylcholine by directly interacting with cholinergic receptors or increasing acetylcholine availability. It classifies cholinergic agonists and anticholinesterases. Cholinergic agonists include acetylcholine and analogs like methacholine and carbachol. Anticholinesterases reversibly or irreversibly inhibit the enzyme acetylcholinesterase, leading to acetylcholine accumulation. Common anticholinesterases discussed are physostigmine, neostigmine, pyridostigmine, and organophosphates. The document provides examples of clinical uses and synthesis for several cholinergic agents.