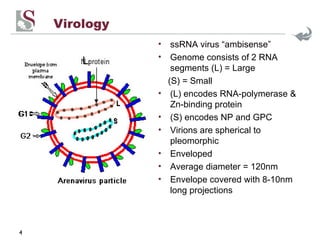
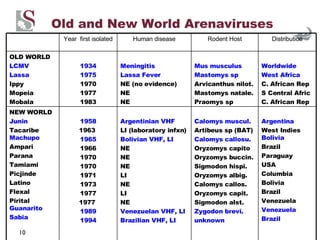
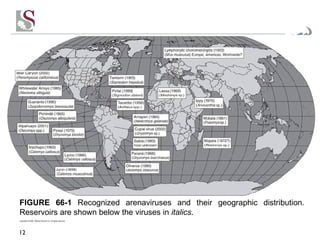
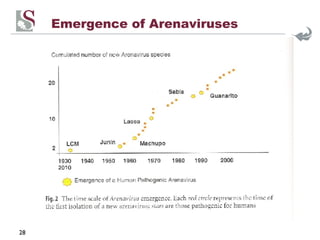
Arenaviruses are single-stranded RNA viruses that are transmitted between rodents and from rodents to humans, causing diseases like Lassa fever. There are over 20 recognized arenavirus species divided into Old World and New World groups based on their rodent hosts and geographic locations. Arenaviruses are enveloped viruses that cause chronic, lifelong infections in rodents and can be transmitted between rodents or from rodents to humans through contact with infected excretions. They have recently been emerging in new locations through mutations, reassortments, or recombinations of their genome segments.