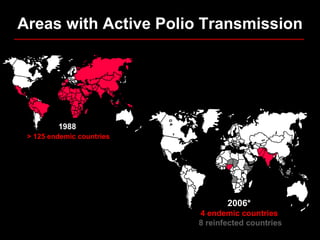
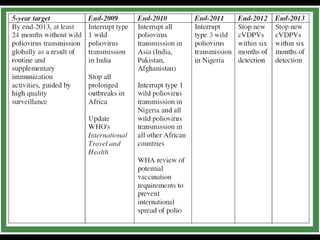
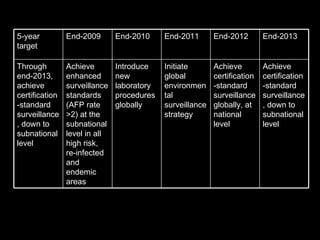
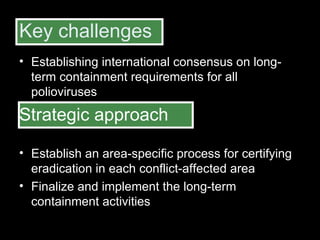
The document outlines the goals and strategic plan of the Global Polio Eradication Initiative from 2009-2013. The goal is to ensure no child is paralyzed by wild or vaccine-derived poliovirus. The plan focuses on interrupting wild poliovirus transmission in the remaining endemic countries, ensuring global surveillance, achieving certification of eradication, preparing for a post-OPV world, and restructuring the initiative for the post-eradication phase. Key strategies include aggressive supplemental immunization activities, optimizing OPV delivery and outbreak response, and establishing high quality surveillance globally.