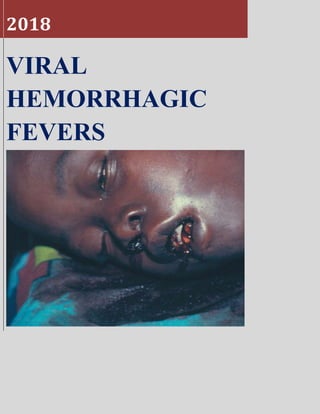
VIRAL HEMORRHAGIC FEVER
- 2. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 1 By OLAOLUWA CHRISTIAN AMARVI
- 3. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 2 OUTLINE Introduction Etiology Epidemiology Pathogenesis and Morphology Clinical features and Diagnosis Prevention and Control Management and Treatment Complications References
- 4. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 3 INTRODUCTION Viral hemorrhagic fevers (VHFs) are a group of febrile illnesses caused by enveloped RNA viruses from several viral families. The concept of viral hemorrhagic fever (VHF) was originated in the 1940s by Soviet investigators, who were studying hantaviral hemorrhagic fever (HF) with renal syndrome and it describes a life threatening multisystem disease syndrome characterized by fever, malaise, vomiting, mucosal and gastrointestinal (GI) bleeding, edema, hypotension and shock. Viral hemorrhagic fevers can be associated with mortality rates that range from less than 1% to over 80%, depending on the specific viral HF and may be caused by over 30 viruses belonging to four taxonomic families: Arenaviridae, Bunyaviridae, Filoviridae, and Flaviviridae. They are all enveloped RNA viruses and are maintained in specific natural cycles involving nonhuman primates, bats, rodents, domestic ruminants, humans, mosquitoes, or ticks, which serve as natural reservoirs or vectors, humans are not usually natural reservoirs except in Dengue virus. Humans can be infected when they come in contact with these natural reservoirs or vectors, human to human transmission is also possible. These viruses can produce a spectrum of illnesses, ranging from a mild acute disease characterized by fever, headache, myalgia, rash, neutropenia, and thrombocytopenia to severe, life-threatening disease in which there is sudden hemodynamic deterioration and shock. Despite the name, absence of hemorrhage should not be used to exclude the diagnosis. Human outbreaks of VHF occur sporadically and treatment is generally supportive. Survivors generally make a full recovery with few sequelae. There is usually no chronic carriage of HF viruses. In most cases, infection is thought to confer lifelong immunity in survivors. Viral hemorrhagic fevers include: Lassa fever Argentine HF Bolivian HF Rift Valley fever
- 5. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 4 Hantavirus pulmonary syndrome Crimean-Congo HF Ebola virus hemorrhagic fever Yellow fever Dengue HF Omsk hemorrhagic fever Kyasanur Forest disease
- 6. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 5 ETIOLOGY Arenaviridae: Includes the genus arena virus which are enveloped viruses with a bisegmented negative-strand RNA genome. They are spread to humans by contact with rodent excreta, urine and other forms of excretions. Human to human transmission is also possible with possible nosocomial outbreaks with Lassa and Machupo presenting a risk for healthcare worker. Includes Lassa virus and the rare South American hemorrhagic viruses. Lassa virus is the most clinically important in this group, Lassa fever was first seen in Lassa, Nigeria in 1969 and its natural host is a rodent (Mastomys natalensis) whose virus-containing excreta is the source of transmission. Virus Family Disease (Virus) Natural Distribution Usual Source of Human Infection Incubation Period(Days) Arenavirus Lassa fever Africa Rodent 5-16 (2-21) Argentine HF (Junin) South America Rodent 7-14 Bolivian HF (Machupo) South America Rodent 9-15 Brazilian HF (Sabia) South America Rodent 7-14 Venezuelan HF (Guanarito) South America Rodent 7-14
- 7. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 6 Bunyaviridae: Comprises of three genera; Phlebovirus(Rift valley fever), Nairovirus( Crimean-Congo HF) and the hantaviruses( about 20 of them). Most are characterized with abrupt onset of symptoms (new world hanta viruses are the exception). mode of transmission mainly by rodents or ticks. Human to human transmission is seen only in Crimean-Congo Hemorrhagic fevers and hantavirus pulmonary syndrome caused by Andes virus. Virus Disease Principal vector/reservoir Geographical distribution Incubation period (days) Old world hantaviruses: Hantaan, Seoul, Puumala, Dobrava- Belgrade, others HF with renal syndrome Rodents Variable depending on the specific virus 9-35 New world hantaviruses: Sin Nombre, Andes, Laguna Negra, others Hantavirus pulmonary syndrome Rodent Variable depending on the specific virus. 7-35 Rift Valley fever Rift Valley fever Domestic livestock/mosquitoes (Aedes and others Sub-Saharan Africa, Madagascar, Saudi Arabia, Yemen 2-5 Crimean-Congo HF Crimean- Congo HF Wild and domestic vertebrates/tick (primarily Hyalomma species) Africa, Balkans, southern Russia, Middle East, India, Pakistan, Afghanistan, western China 3-12
- 8. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 7 Filoviridae: The filovirus infections; Marburg and Ebola HFs, are the most severe and feared of all viral HFs. The Filoviridae are comprised of six species of Ebolavirus and one Marburgvirus. The natural reservoir for the filoviruses are the fruit bats with human infection likely from inadvertent exposure to infected bat excreta or saliva. Human to human transmission of filoviruses is high. With fatality rates that are usually high but vary among specific strains of the viruses. Ebola virus: Ebola virus was first described in 1976 after outbreaks of a febrile, rapidly fatal hemorrhagic illness were reported along the Ebola River in Zaire (now the Democratic Republic of the Congo) and Sudan. Sporadic outbreaks have continued since that time, usually in isolated areas of central Africa. An outbreak in Kikwit, Zaire, in 1995 led to 317 confirmed cases, with an 81% mortality rate. Two thirds of the cases were in health care workers caring for infected individuals. An outbreak in Uganda in late 2000 resulted in 425 cases and claimed 225 lives. The largest Ebola outbreak to date occurred in West Africa from 2014 to 2016. This outbreak primarily occurred in Guinea, Sierra Leone, and Liberia, with >28,000 cases and >11,000 deaths. As a result of this outbreak, several sporadic cases of imported Ebola virus disease also occurred in industrialized nations, including the United States, the United Kingdom, Spain, and Italy. Fruit bat believed to be the natural reservoir of Ebola and Marburg viruses Flaviviridae: The two most important are Yellow fever and Dengue fever hemorrhagic viruses. Both are mosquito-borne viruses It also includes two viruses in the tick-borne encephalitis group that cause VHF: Omsk hemorrhagic fever virus and Kyasanur Forest disease virus.
- 9. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 8 Aedes aegypti the vector for yellow fever and dengue viruses Virus Disease Natural vector/reservoir Geographic distribution Incubation Period (days) Yellow fever Yellow fever Monkey/mosquito (Aedes aegypti, other Aedes and Haemagogus spp.) Sub-Saharan Africa, South America up to Panama. 3-6 Dengue Dengue HF Human/mosquito (Aedes aegypti and albopictus Tropics and subtropics worldwide 3-15 Kyasanur Forest disease Kyasanur Forest disease Vertebrate (rodents, bats, birds, monkeys, others)/tick (Haemophysalis species and others) Karnataka State, India; Yunnan Province, China; Saudi Arabia. 3-8 Omsk HF Omsk HF Rodent/ticks (primarily Dermacentor and Ixodes species) Western Siberia 3-8 .
- 10. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 9 EPIDEMIOLOGY Taken together, the viruses that cause HF are zoonotic. Consequently, the endemic areas for the various viral HFs are limited to the distribution of their mammalian reservoirs and/or arthropod vectors. Although modern-day ease of travel has made it possible for cases of viral HF to be seen throughout the globe, imported cases remain quite rare. This partly accounts for the higher incidence of VHFs in the tropics. Emphasis will be laid on some VHFs common in our parts of the world; Yellow fever (YF), Lassa fever (LF), Dengue HF (DHF), Ebola HF (EHF) / Ebola virus disease (EVD). Lassa fever: About 80% of people who become infected with Lassa virus have no symptoms. 1 in 5 infections result in severe disease. It results in about 300,000 to 500,000 cases and causes about 5,000 deaths yearly. Outbreaks have been seen in Nigeria, Liberia, Sierra Leone, Guinea and the Central African Republic. The most recent outbreak in Nigeria occurred from 1st January to 22nd July 2018 with at least one confirmed case in Edo, Ondo, Bauchi, Nasarawa, Ebonyi, Anambra, Benue, Kogi, Imo, Plateau, Lagos, Taraba, Delta, Osun, Rivers, FCT, Gombe, Ekiti, Kaduna, Abia and Adamawa states, about 120 deaths in confirmed cases and 10 in probable cases with a Case Fatality Rate in confirmed cases of 25.7% according to Nigeria Centre for Disease control (NCDC).
- 11. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 10 2018-Lassa fever outbreak in Nigeria Ebola virus: The most recent outbreak occurred in Democratic Republic of Congo with about 54 cases including 33 deaths. The largest outbreak to date was the epidemic in West Africa, which occurred from December 2013 to January 2016 with 28,616 cases and 11,310 deaths.
- 12. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 11 West African ebola virus disease epidemic (2013-2016)
- 13. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 12 Yellow Fever: In 2013, yellow fever resulted in about 127,000 severe infections and 45,000 deaths, with nearly 90% of these occurring in African nations. Nearly a billion people live in an area of the world where the disease is common.Yellow Fever was described in 1648, but was isolated in 1975. It is common in tropical South America and Africa but not in Asia. It is endemic in areas where there is reduced immunity to it abundance of its natural vector. 2017
- 14. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 13 PATHOGENESIS AND MORPHOLOGY Viral hemorrhagic fevers are characterized by a sudden and severe shock like syndrome in fatal cases, suggesting that inflammatory mediators may play a determining role in the disease pathogenesis.
- 15. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 14 Fatal hemorrhagic fever viral infections are generally characterized by high viraemia and immunosuppression. The primary defect in patients with viral hemorrhagic fever is that of increased vascular permeability. Viral hemorrhagic infections are characterized by deleterious changes in lymphoid tissues and defects in coagulating system. Another common feature among these viruses is that all hemorrhagic fever viruses appear to target and impair the cells that play the most critical roles in initiating the antiviral immune response.
- 16. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 15 General Mechanism of Pathogenesis Dissemination of virus due to suppressed responses by the antigen presenting cells. Prevention of antigen specific immune responses. Apoptosis of lymphocytes. Through infected macrophages when they interact with toxic cytokines leading to diapedesis and coagulation deficiency.
- 17. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 16 Pathogenesis of Ebola Virus The first step for viral life cycle is entry into the host cell, and is a major and multifacet process although the mechanism is poorly understood, cellular entry involves uptake by macro- pinocytosis like mechanism Filovirus has a uniform filamentous shape with a RNA gene that codes for seven protein. Nucleoprotein Vp 35 (polymerase cofactor) Vp 40 (matrix protein)
- 18. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 17 Gp (glycoprotein) Vp 30 (transcription activator) Vp 24 (secondary matrix protein) DNA dependent RNA polymerase . Virus spreads from initial infection site to the primary target sites which are macrophages, dentritic cells, endothelial cells, liver cells and koffer cells. The viral hemorrhagic precursor is cleaved in the trans-golgi network into two glycoproteins; Gp1 and Gp2. Gp1 binds to the host receptor, while Gp2 mediates fusion of viral envelope with host cell membrane. Within the host cell, the virus synthesizes viral proteins that are released. Gp1, Gp2 and Vp40 which are viral particles activates endothelial cells to express intercellular adhension molecules (ICAM-1), vascular cellular adhesion molecule (VCAM-1) and E-selectin which all together increases vascular permeability and are majorly seen within local blood vessels at site of inflammation. This effect is further exacerbated with TNF-α hence, decreasing endothelial barrier function by macrophage infected mononuclear phagocytes. Innate immunity which response to viral infection are also pathological. Viral protein also include innate immunity to express genes for IL-6,IL-8, IL-1(β), IL-18 which are responsible for acute phase reaction, mostly fever. Surface protein form specific for ebola bring about cytopathic effects on endothelial cells which include rounding of cells, increasing membrane permeability and detatchment. Hence, increased vascular permeability.
- 19. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 18 In cytokines storm, some of the chemicals released are antiviral and help in immunologic reaction. Viral proteins inhibit the production of antiviral cytokines, IFN-type1. Hepatic injury caused by direct cytopathic effect on infected hepatocytes and kuper cells leading to deficiency in hepatic synthesis of coagulation factors. This brings about the hemorrhage seen in patients infected with the virus presenting with hepatocellular lesions. Decreased CD4 and CD8 lymphocytes, associated with a large increase in Fas-causing apoptosis hence leukopenia particularly lymphopenia leading to reaction in immunity. Multifocal necrosis mainly a cause of DIC associated with fibrin thrombine deposits within blood veassels Pathogenesis of this condition may lead to depletion of protein C levels which in the coagulatiom cascade is involved in fibrinolysis by inactivating factors Va and Viiia. Replication cycle of Ebola virus
- 20. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 19 Pathogenesis of Lassa Fever Lassa fever is an acute multiorgan infection. The incubation period vary between 5 and 21 days. Virus enters the human body through the blood stream, lymph vessels, respiratory tracts and/or digestive tracts mainly by endocytosis. The receptor used for cell entry is alpha-dystroglycan, a highly conserved and ubiquitiously expressed cells of surface receptor for ECM proteins. Virus can effect when different kinds of cells: Macrophages, dendtritic cells, megakaryocytes,hepatocytes, endothelial cells, renal epithelial cells, astroglia. Once within the cell (endocytosis), the viruses are rapidly delivered to endosomes via vesicular trafficking although one that is largely dependent on the small GT phases. Low pH environment triggers pH dependent fusion and release RNP (viral ribo-nucleoprotein) complex into the cytoplasm. Viral RNA is unpacked, and replication and transcription initiates in the cytoplasm. Generally when a pathogen enters into a host, innate defence system recognizes the PAMPs (pathogen associated molecular patterns) and activates immune responses. In the cytoplasm, dsRNA receptors such as RIG-1 (retinoic acid-inducible gene 1) and MDA-5 (melanoma deifferentiation associated gene 5) detects dsRNAs and initiates signaling pathwas that results in translocation on IRF-3 (interferon regulator factor) and other transcription factors to the nucleus, which activates adaptive immunity. Nucleoprotein (NP) encoded in Lassa virus is essential in viral replication and transcription factors, but it also suppresses host innate IFN response by inhibiting translocation of IRF-3. This enables it evade host immune response. Upon entry, Lassa virus infects almost every tissue in the human bod. It starts with the mucosa, intestine, lungs and urinary system, and then progresses to the vascular system.
- 21. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 20 Nucleprotein (NP) encoded in Lassa virus is essential in viral replication anf transcription, but it also suppresses host innate IFN response by inhibiting translocation of IRF-3. Lassa fever is not considered to be associated with coagulation dysfunction eg DIC. However, since megakaryocytes are affected, platelet production is impaired as their functionality is severely compromised. There is infection triggered induction of uncontrolled cytokine expression mostly seen in sepsis. Virus induced immunosuppression may also be involved in the pathogenesis of severe Lassa fever. Lassa fever is not considered to be associated with coagulation dysfunction eg DIC. However, since megakaryocytes are affected, platelets production is impaired as their functionality is severely compromised. YELLOW FEVER The viremic phase of yellow fever are day 3 to 6. Replication begins at the site of inoculation. The virus is transferred from the infected female mosquito’s salivary gland by means of saliva introduced into a bite wound during a blood meal. It replicates in local tissues and regional lymph nodes, the virus can infect a feeding mosquito during the initial 3 to 4 days of illness. No human to human transmission is known.
- 22. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 21 councilman bodies After the virus has entered the blood stream, hematogenous spread to the bone marrow, kidney, liver(probably indirectly via kupffer cells). This leads to eosinophilic degradation of these cells and the release of cytokines. Apoptotic masses known as councilman bodies appear the cytoplasm of the hepatocyte. Fatality may follow when the cytokines storm, shock, and multiple organ failure occur .
- 23. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 22 MORPHOLOGICAL CHANGES IN VIRAL HEMORRHAGIC FEVERS Viral hemorrhagic fever cause changes in vital organs of the body such as liver, kidney, spleen, lungs and blood vessels. Hemorrhagic fever viruses cause changes in vital organs of the body such as liver, kidney, spleen,lungs and blood vessels. Morphological Changes in Ebola Virus Disease. LIVER: There is moderate hyperemia and marked edema in the centre of lobules with atrophy and dissociation of the liver cell cords in the area. Liver cells loaded with fat droplets, while some undergo necrosis.The portal tracts are enlarged and rather intensely infiltrated with lymphoid cells. Electron microscopy: Hepatocytes showed enlarged mitochondria without cristae containing coarse granules or being empty. Many empty vacuoles indicating fat droplets in cytoplasm were seen. The spaces of Dissé were considerably enlarged. Frequently the plasmatic membranes of hepatocytes were not recognizable. Microvilli were absent. Inside the cytoplasm of altered hepatocytes meander like inclusions of 1 microm as well as single filamentous particles were found. However, inclusion bodies of nucleocapsids with regular formation were not present. The extracellular space contained virus particles and nucleocapsids not to be distinguished from Marburg virus particles. Ebola (Maridi) virus in human liver,
- 24. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 23 Bone marrow: twin nucleus and vacuolization of metamyelocyte . SPLEEN: There was marked hyperemia and cellular depletion of the red pulp and atrophy of lymphoid follicles MYOCARDIUM: There was general, rather proteinaceous edema of locally varying degree of the interstitial connective tissue with accumulations of inflammatory cells similar to those found in the interstitial connective tissue of the liver and kidney. LUNG: The alveoli are focally atelectative. The alveolar walls are generally and moderately thickened due to an increase in cellularity and deposit of fibrinoid within and adjacent to the surface of the alveolar wall. KIDNEY: The glomerula were inconspicuous. The epithelial cells of the tubules, particularly of the proximal portion of the nephron, exhibited varying degrees of granular, hydropic, and fatty degeneration and - focally necrosis and desquamation. The Bowman's space at the glomeruli and the lumen of the tubules were irregularly filled with amorphous proteinaceous precipitate. -ocal cellular infiltrations - analogous to those found in the portal tracts 'if the liver - were seen around blood vessels, particularly at the corticolymphocytes had its peak between the 6th and 10th day, though these features could be observed in smears from 3rd to 24th day of illness.
- 25. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 24 MORPHOLOGICAL CHANGES IN LASSA FEVER. GROSS PATHOLOGICAL FINDINGS 1) Visceral congestion. 2) edema of soft tissues. 3) petechiae especially in the GIT. 4) pleural effusion and ascites. MICROSCOPY LIVER: The liver is the main target organ in the human lassa infection . The cytoplasm becomes focally condensed prodeucing an eosinophilic cytoplasm inclusion with pkynosis or disappearance of nuclei. Focal hepatic necrosis are also scattered throughout the lobules without zonal distribution.
- 26. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 25 The reticulum framework of the liver remains intact. The amount of inflammation is small and not released to the extent of hepatocellular damage. Leukocytes and mononuclear cells are scattered through the sinusoids Lassa virus in human liver. Distinction between ribosomes (within the virus particles) and other granules, such as glycogen (arrows), is necessary for virus identification in a background of pathological tissue
- 27. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 26 HEART: congestion, slight interstitial edema and nonspecific epicardial infiltrates are present. Interstitial pneumonitis with mononuclear cells and megakaryocytes are also noted. SPLEEN: atrophy of the white pulp and deposition of amorphous eosinophilic material in the white pulp are present in several cases. Infiltration of the intima of the splenic veins by lymphoid cells can occur. GIT: Petechiae and evidence of chronic inflammation especially in the mucosa KIDNEY: There are occasional focal tubular and glomerular necrosis.congestion autolysis and hyaline or pigment casts are also present. Morphological changes seen in yellow fever Yellow fever is characterized by hepatic dysfunction, renal failure, coagulopathy and shock LIVER: The midzone of the liver lobule is principally infected,with sparing of the cells bordering the central vein and portal tracts. Viral antigen localizes to the midzone indicating that this is the site of direct viral injury. Very high viral loads have been found in liver and spleen of fatal cases. Injury to the hepatocytes is characterized by eosinophilic degeneration with condensed nuclear chromatin. Liver cell death is due to apoptosis. There is no disruption of the reticular architecture of the liver. KIDNEY: Renal damage is characterized by eosinophilic degeneration and fatty change of renal tubular epithelium without inflammation.These findings are belived to be as a result of both directional injury and nonspecific changes due to hypotension and hepatorenal syndrome. HEART: focal injury to the myocardium characterized by cell degeneration and fatty change, is the result of viral replication. The late phase of the virus is characterized by circulatory shock. The underlying mechanism may be cytokine dysregulation, as in the sepsis syndrome. Autopsy of patient who died of yellow fever show cerebral edema, probably as a result of microvasular dysfunction.
- 28. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 27 CLINICAL PRESENTATION Viral hemorrhagic fever is seen in both genders and all age groups though there could be occupational predisposition, with a spectrum from relatively mild or even asymptomatic infections to severe vascular, permeability with shock, multi-organ system failure and death. Although the clinical presentation may differ for each viral hemorrhagic fever as it progresses, in most cases it is not possible to distinguish the various syndromes at presentation. Incubation period ranges from days to weeks depending on the infecting virus. Illness typically begins with initial nonspecific signs and symptoms such as fever , general malaise , anorexia, headache, myalgia ,sore throat, arthralgia, chest or retrosternal pain and lumbosacral pain. Orthostatic hypotension is common. Conjunctival injection or hemorrhage without itching, drainage or rhinitis is frequent. Various forms of skin rash, including morbilliform, maculopapular, petechial and ecchymotic, may be seen, depending on the specific viral HF. Radiographic and electrocardiographic findings are generally nonspecific and correlate with the physical examination. Gastrointestinal signs and symptoms follow in the first few days of illness, including nausea, vomiting, epigastric and abdominal pain, abdominal tenderness, diarrhea and constipation. Diarrhea may become bloody in the later stages of the disease. In severe cases, towards the end of the first week of illness, patients progress to vascular instability that may be manifested by facial flushing, oedema, bleeding, hypotension, shock, and proteinuria . Significant internal bleeding from the gastrointestinal tract may occur even in the absence of external haemorrhage . Central nervous system manifestations, including disorientation, tremor, gait anomalies, convulsion and hiccups may be noted in the end stages of some VHFs. Rectal bleeding
- 29. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 28 DIAGNOSIS DIFFERENTIAL DIAGNOSIS Many infectious and noninfectious diseases can mimic VHFs especially in the initial stages resulting in a wrong diagnosis. Malaria and bacterial septicemia, including Meningococcaemia are the greatest culprits. Possibility of co-infection should also be considered. Features making VHF less likely include: Haemorrhage in the first few days of illness. Conjunctival injection/sub-conjunctival haemorrhage accompanied by itching, discharge or rhinitis. The itching, discharge and rhinitis make an upper respiratory tract infection more likely Jaundice on presentation (except YF). Prominent pulmonary symptoms. Response to antibiotics. Differential diagnosis may include: •Inluenza •Viral hepatitis (hepatitis A, B, E, EBV and CMV) •Herpes simplex or varicella-zoster (fulminant) •Alphavirusinfection (chikungunya, o’nyong-nyong) •Typhoid fever •Bacillary dysentery •Meningococcaemia and many more CLINICAL DIAGNOSIS The nonspecific clinical manifestations of most viral HFs make a clinical diagnosis and detection of single cases extremely difficult, especially early in the course of the disease when haemorrhage and other more identifiable manifestations are usually absent. History of illness, detailed epidemiological history, physical examination, preliminary basic laboratory results are critical in initial consideration of the diagnosis. A diagnosis of viral HF should be considered in patients with a clinically compatible syndrome who fulfills any epidemiologic linkage criteria.
- 30. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 29 LABORATORY DIAGNOSIS Some tests are indicative in the clinical diagnosis of viral hemorrhagic fevers and they include: Leukocyte count : initially moderate leukopenia then leukocytosis with left shift comes later (granulocytosis more suggestive of bacterial infection) Haemoglobin and haematocrit : Haemoconcentration (especially noted in haemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome Platelet count: Mild-to-moderate thrombocytopenia BUN/creatinine AST, ALT, lactate : Usually increased, especially in severe disease; AST > ALT; Blood gas: Metabolic acidosis may be indicative of shock and hypoperfusion Coagulation studies (PT/PTT, fibrinogen, FDP, D-dimer): DIC common in Ebola, Marburg, Lujo virus, Crimean-Congo HF and New World arenavirus infections. Urinalysis : Proteinuria common; Haematuria may be occasionally noted; Sediment may show hyaline-granular casts and round cells with cytoplasmic inclusions Blood culture: Useful early to exclude viral HF and later to evaluate for secondary bacterial infection; Blood should be drawn before antibiotic therapy is instituted. Stool culture: Useful to exclude viral HF (in favour of haemorrhagic bacillary dysentery Thick/thin blood smears Rapid testfor malaria Assay for Salmonella Mainstay of diagnosis (confirmatory) done in specialized regional laboratories include: (enzyme-linked immunosorbent assay) -PCR(reverse transcriptase polymerase chain reaction) ll culture (immunofluorescent antibody test): not as routinely sensitive or specific and is more subjective in its interpretation, varying with the experience of the technician. staining of formalin-fixed tissue: skin, liver, spleen (Post-mortem diagnosis)
- 31. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 30 PREVENTION AND CONTROL Vaccine is present for Argentine hemorrhagic fever and yellow fever. Other than these, there are no vaccines present,which can protect a person from viral hemorrhagic fever. Therefore,it is important to observe the following preventive methods avoidance of contact and eradication of vectors Education of the general public on disease symptoms and transmission and management. Maintenance of general sanitation Vaccines are available for some of the virus e.g yellow fever 17-d vaccine Identify suspected cases and quarantine Identify patient contact and travel history Infected patients should be taken to a well facilitated isolation center Wearing of protective gear by medical personnel Safe burial practices for dead patient Personal protective equipment for the management of VHF
- 32. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 31 MANAGEMENT AND TREATMENT Treatment of viral hemorrhagic fever can be divided into general supportive measures, antiviral drugs,antibody therapy GENERAL SUPPORTIVE MEASURES Treatment of the viral hemorrhagic fever generally follows the guidelines for the management of septic shock. Where possible patients with viral hemorrhagic fever should be treated in an intensive care unit since sever microvascular instability, often complicated by vomiting, diarrhea, decreased fluid intake , may require continuous monitoring and aggressive fluid replacement. Because of the risk of bleeding at insertion sites, intravascular hemodynamic devices are contraindicated. Intravascular and subcutaneous injections should be avoided due to the risk of haematoma. FLUID MANAGEMENT; fluid management in viral hemorrhagic fever poses a particular challenge. Aggressive fluid replacement is warranted and may prevent shock and DIC. ANTIVIRAL THERAPY; the antiviral drugs are useful in the management of viral hemorrhagic fever is ribavirin, ribavirin has been shown to reduce mortality in hemorrhagic fever with renal syndrome if administered within four days of onset. Pain control and ulcer prophylaxis; acetaminophen, tramadol, opiates and or other analgesics should be used for pain control. Salicylates and non- steroidal anti –I nflammatory drugs should be avoided due to the risk of bleeding.prophylactic therapy for stress ulcers with H2 receptor antagonists is appropriate. Antibody therapy ; with the exception of treatment of argentine hemorrhagic fever, this therapy should be reserved for severe and refractory cases when ribavir.in is not an option either because it is not available or because the patient has a viral hemorrhagic fever for which ribavirin is not efficacious, examples; Immune modulators Coagulation modulators
- 33. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 32 COMPLICATIONS OF VIRAL HEMORRHAGIC FEVERS The following complications can arise as a result of viral hemorrhagic fever; Shock DIC- disseminated intravascular coagulopathy Pericarditis Illness induced abortion in pregnant women Hematemesis and bloody diarrhea Altered mental status and cardiovascular collapse [pre-terminal events] In severe cases, hemorrhage exudes from mucous membranes, venipuncture sites, and body orifices. The formation of antigen antibody complexes during recovery may cause acute arthralgias and other symptoms. Progression to multi-organ failure and septic shock Death typically occur between 6 and 16 days Post ebola virus syndrome: The New England Journal of Medicine reports that symptoms include lethargy, joint pains, hair loss, and vision loss, frequently to the point of near blindness, and uveitis
- 34. VIRAL HEMORRHAGIC FEVERS-GROUP 1 PAGE 33 REFERENCES http://wwwn.cdc.gov/ National Notifiable Diseases Surveillance System (NNDSS) Annual reviews of pathology 2014 www.medscape.com/viral hemorrhagic fever Viral Hemorrhagic fevers in Nigeria by Dr T Oricha Viral Haemorrhagic Fevers by Lucille Blumberg Viral hemorrhagic fevers by group 4 2016/2017 Nigerian Centre For Disease Control Robbins and Coltran textbook of Pathology Harsh Mohan- Textbook of Pathology