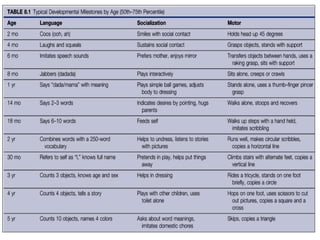
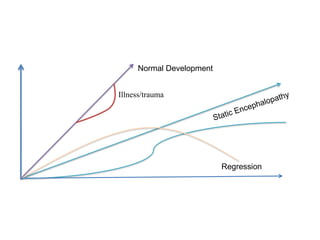
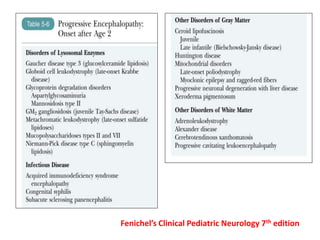
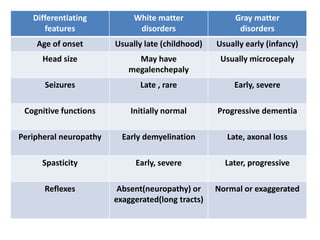
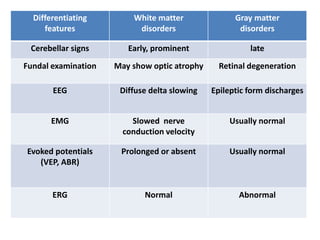
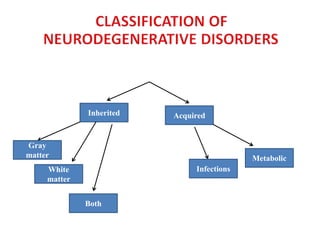
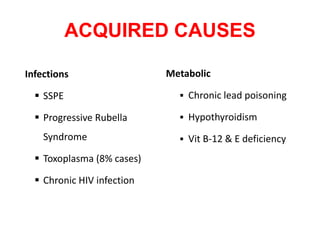
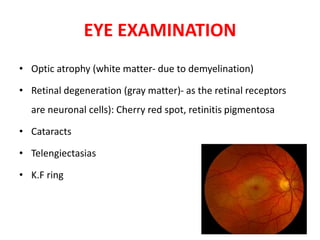
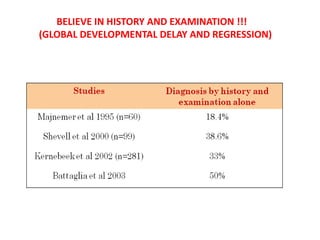
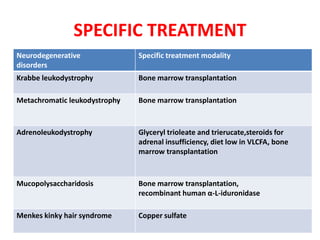
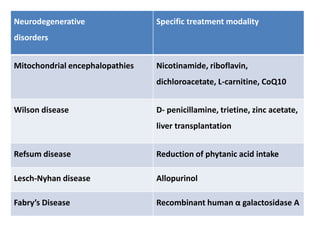
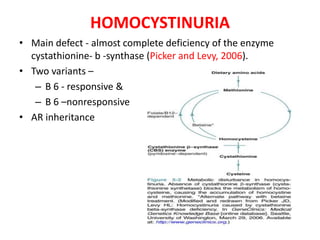
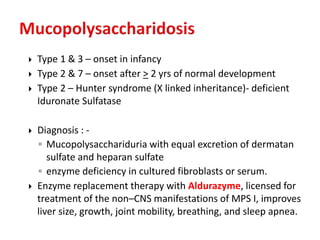
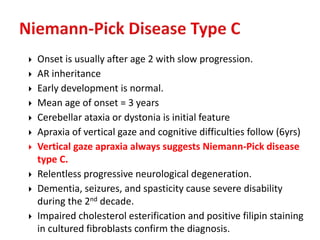
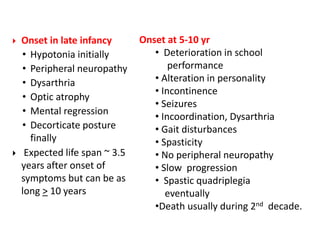
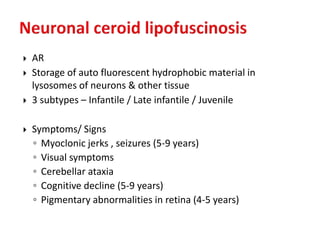
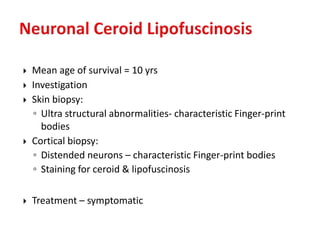
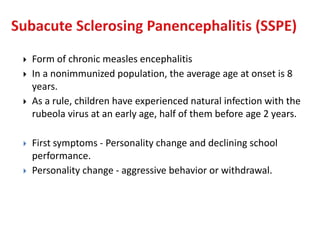
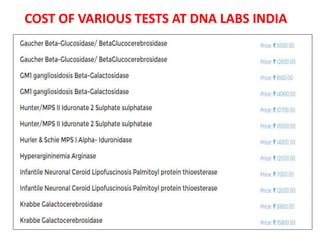
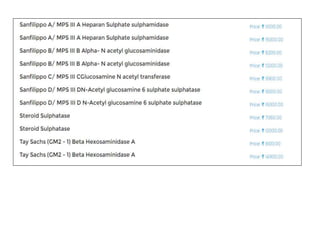
This document discusses the approach to evaluating children presenting with developmental regression. It defines developmental regression as the loss of developmental milestones previously attained, indicating a progressive nervous system disease. The evaluation involves a detailed history, developmental assessment, neurological exam, and targeted investigations to identify underlying genetic, metabolic, or acquired etiologies and guide management. A multidisciplinary approach is emphasized to address developmental delays, seizures, contractures, feeding issues, and provide genetic counseling.