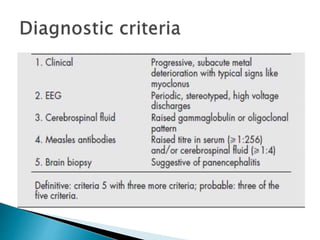
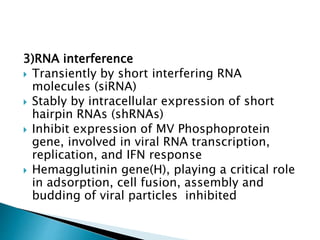
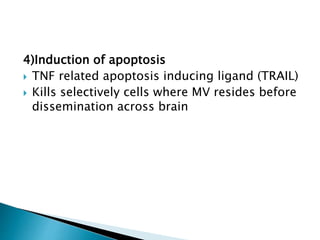
i. Subacute sclerosing panencephalitis (SSPE) is a rare progressive neurological disorder caused by persistent measles virus infection of the brain. It presents with personality changes, myoclonus, rigidity and progressive deterioration.
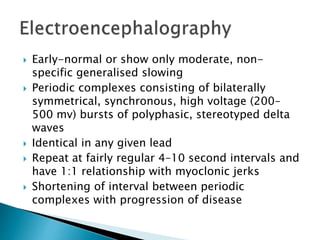
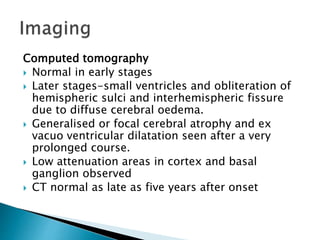
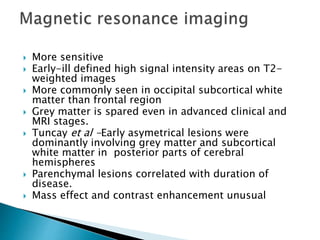
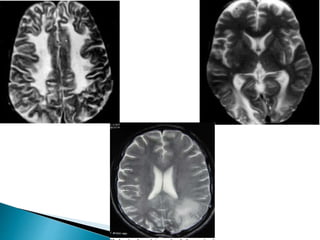
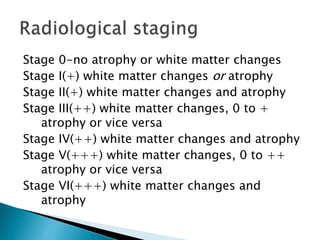
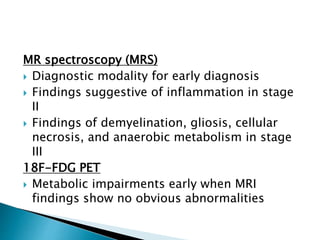
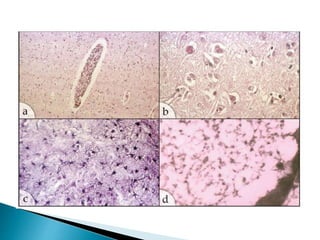
ii. Pathologically, it is characterized by neuronal inclusion bodies containing measles virus antigens. MRI may show non-specific white matter changes while EEG typically shows periodic complexes correlated with myoclonus. There is no cure and treatment is supportive only.
iii. Risk factors include measles exposure before 2 years of age. Prognosis is poor with most patients dying within 3 years, though rare spontaneous remissions occur in 5-6% of