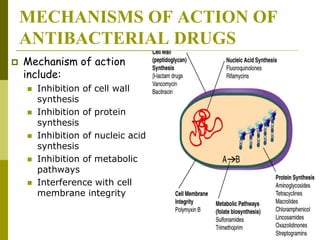
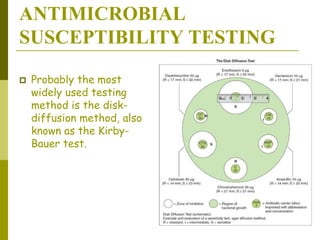
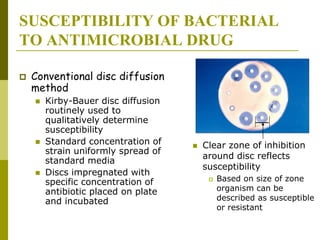
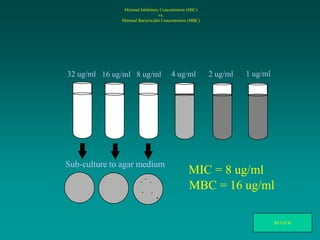
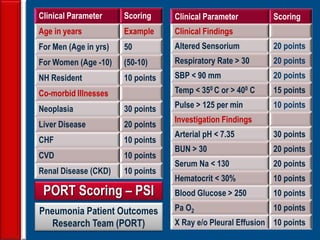
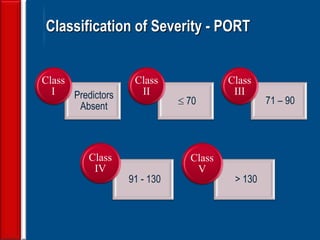
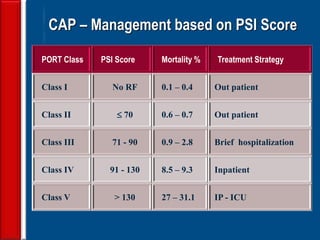
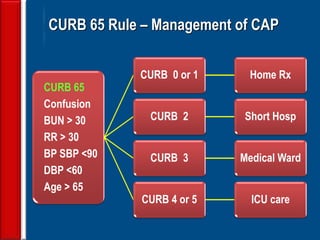
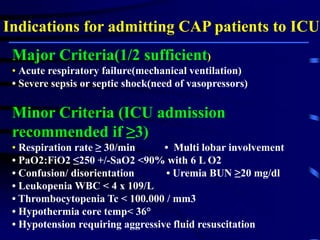
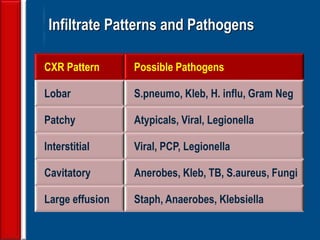
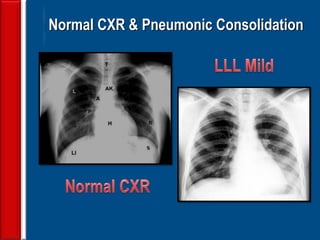
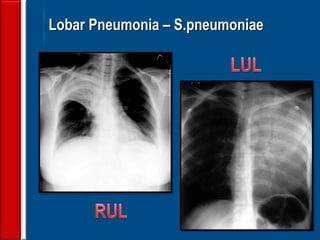
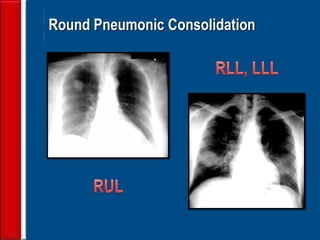
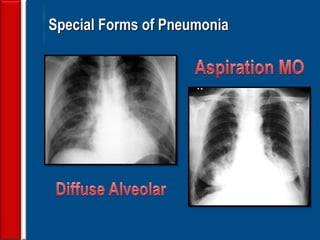
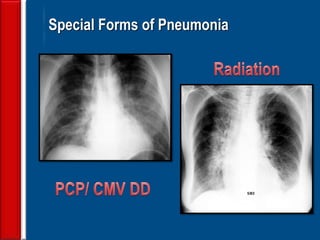
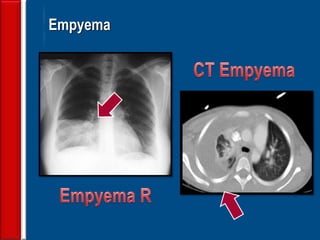
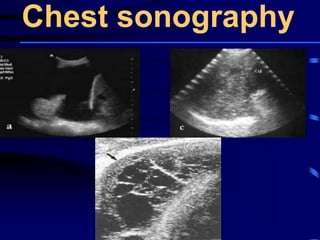
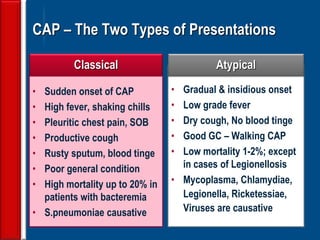
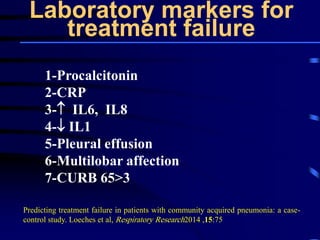
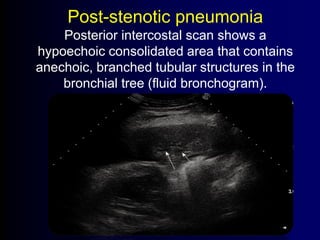
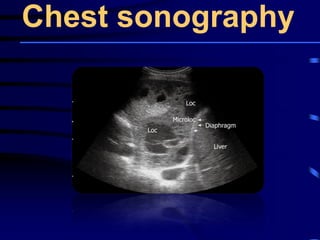
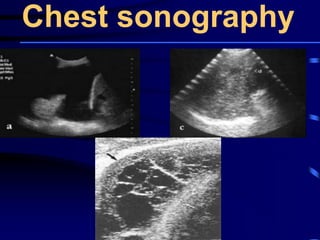
This document discusses antibiotic strategies for community-acquired pneumonia (CAP) and exacerbations of chronic obstructive pulmonary disease (AECOPD). It outlines the mechanisms of action, testing, and spectrum of various antimicrobial drugs. It also describes methods for assessing the severity of CAP cases and determining appropriate treatment and site of care based on severity scores. Empiric antibiotic recommendations are provided for outpatient, inpatient, ICU, and specific pathogen cases of CAP.


















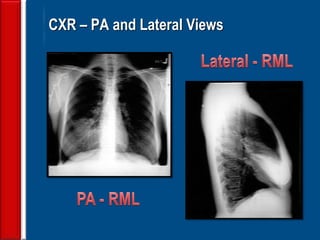
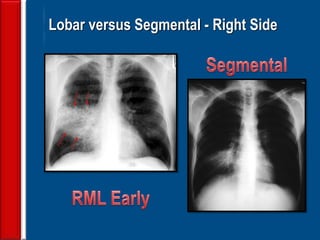
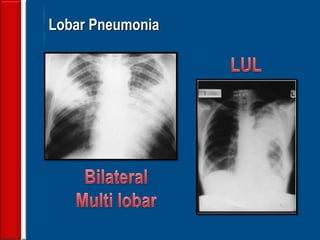
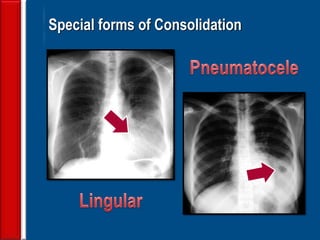

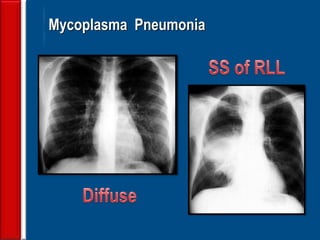
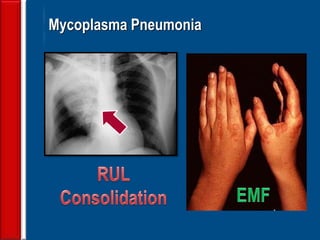
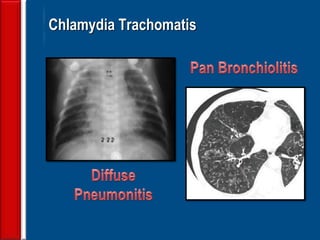
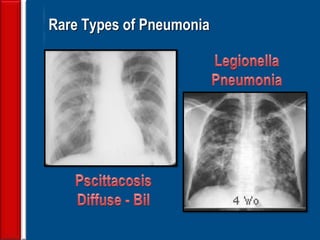
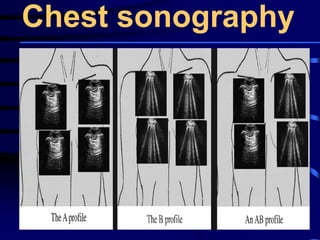
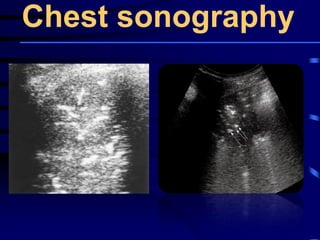
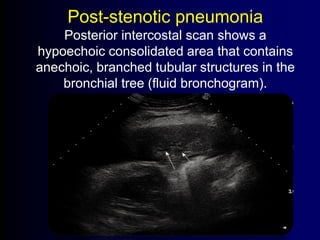
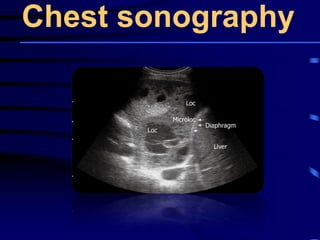






















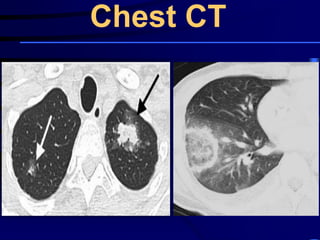
![Evaluating a patient who is not
responding to therapy
◙Repeating the history (including travel and pet
exposures to look for unusual pathogens), chest
radiograph, and sputum cultures, blood cultures, and
urine antigen testing for Streptococcal pneumoniae and
Legionella if not previously done .
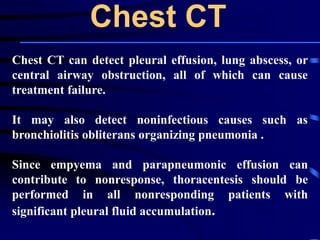
◙If this is unrevealing, then further diagnostic
procedures,, such as chest computed tomography [CT],
bronchoscopy, and lung biopsy can be performed.](https://image.slidesharecdn.com/antibioticstrategyincapaecopd-161221211548/85/Antibiotic-strategy-in-CAP-AECOPD-76-320.jpg)





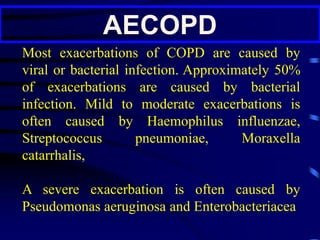


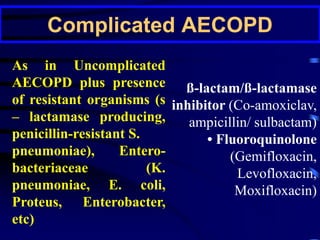


![P. aeruginosa should be considered
in the presence of at least two of the
following [recent hospitalization, frequent
(>4 courses per year) or recent
administration of antibiotics (last 3 months),
severe disease (FEV1 < 30%), oral steroid
use (>10 mg of prednisolone daily in the last
2 weeks)].](https://image.slidesharecdn.com/antibioticstrategyincapaecopd-161221211548/85/Antibiotic-strategy-in-CAP-AECOPD-95-320.jpg)
