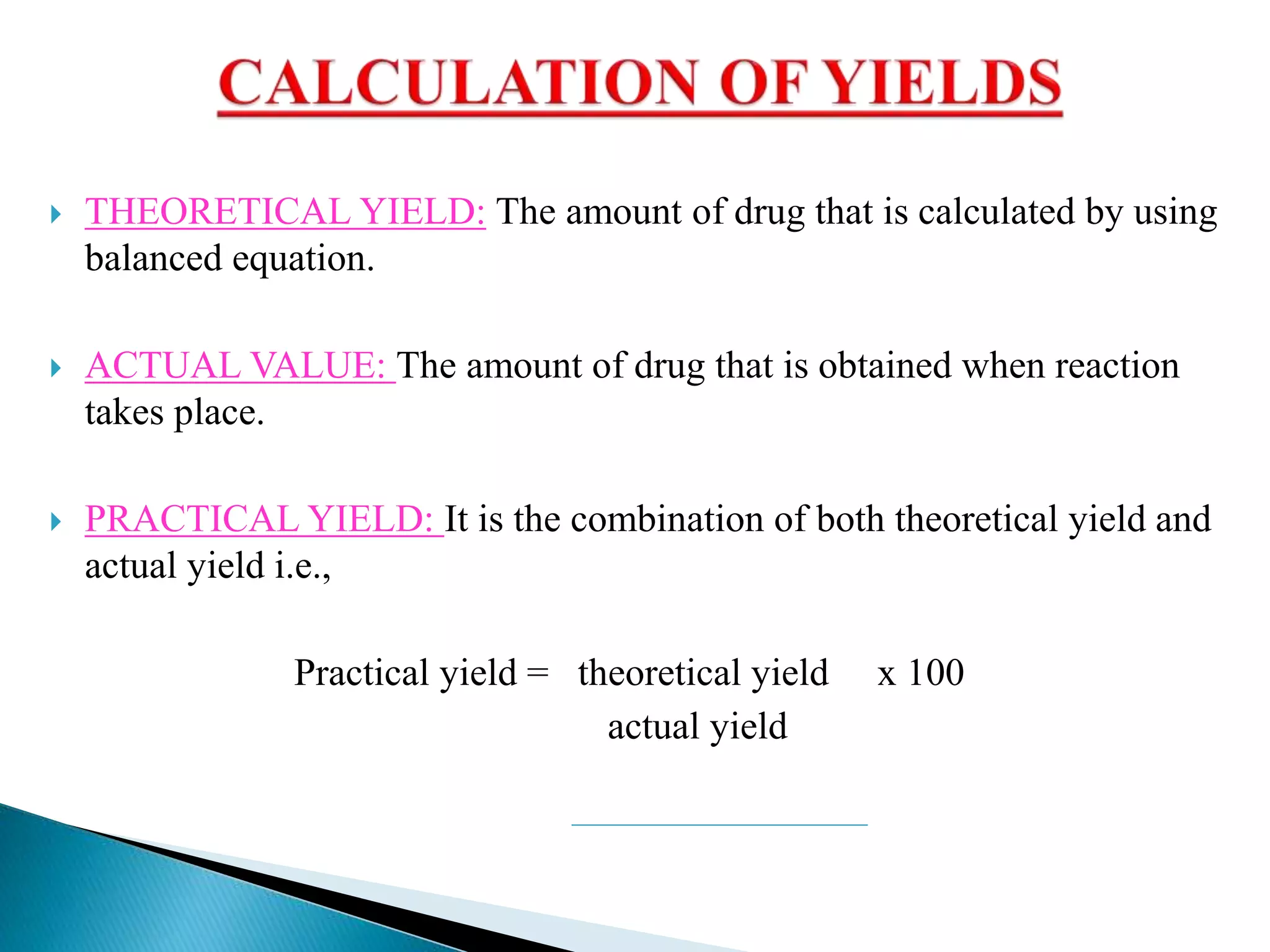
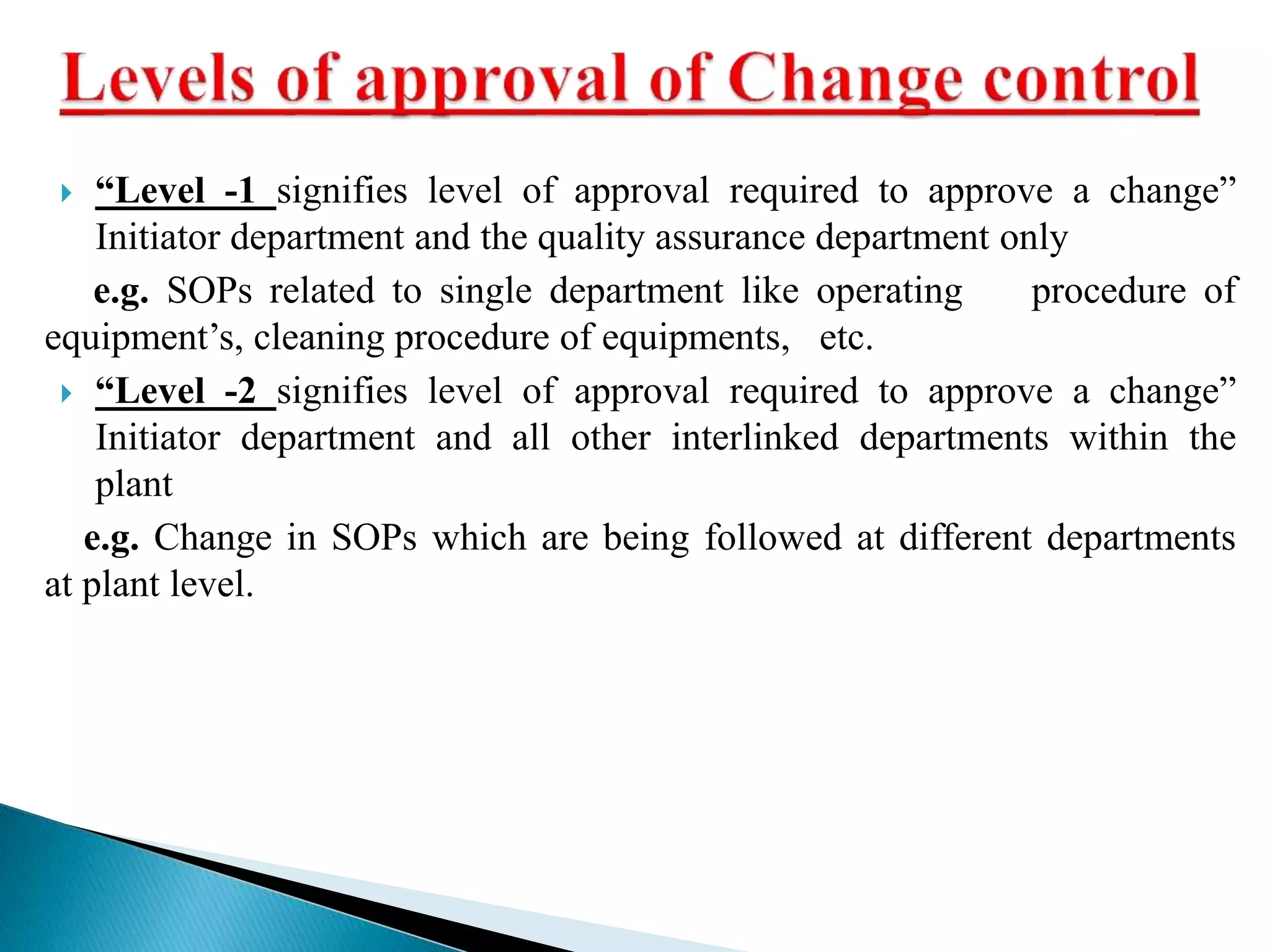
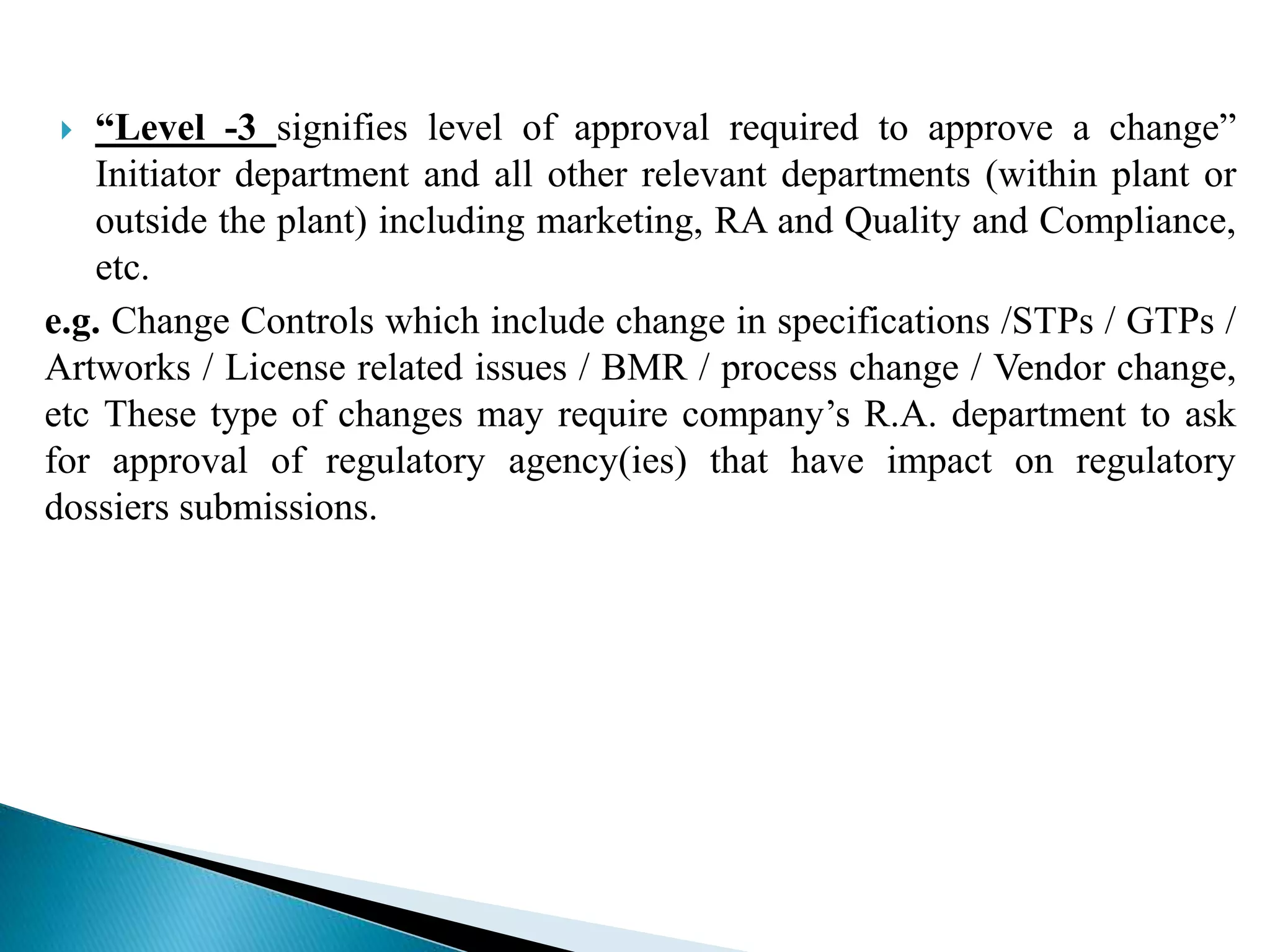
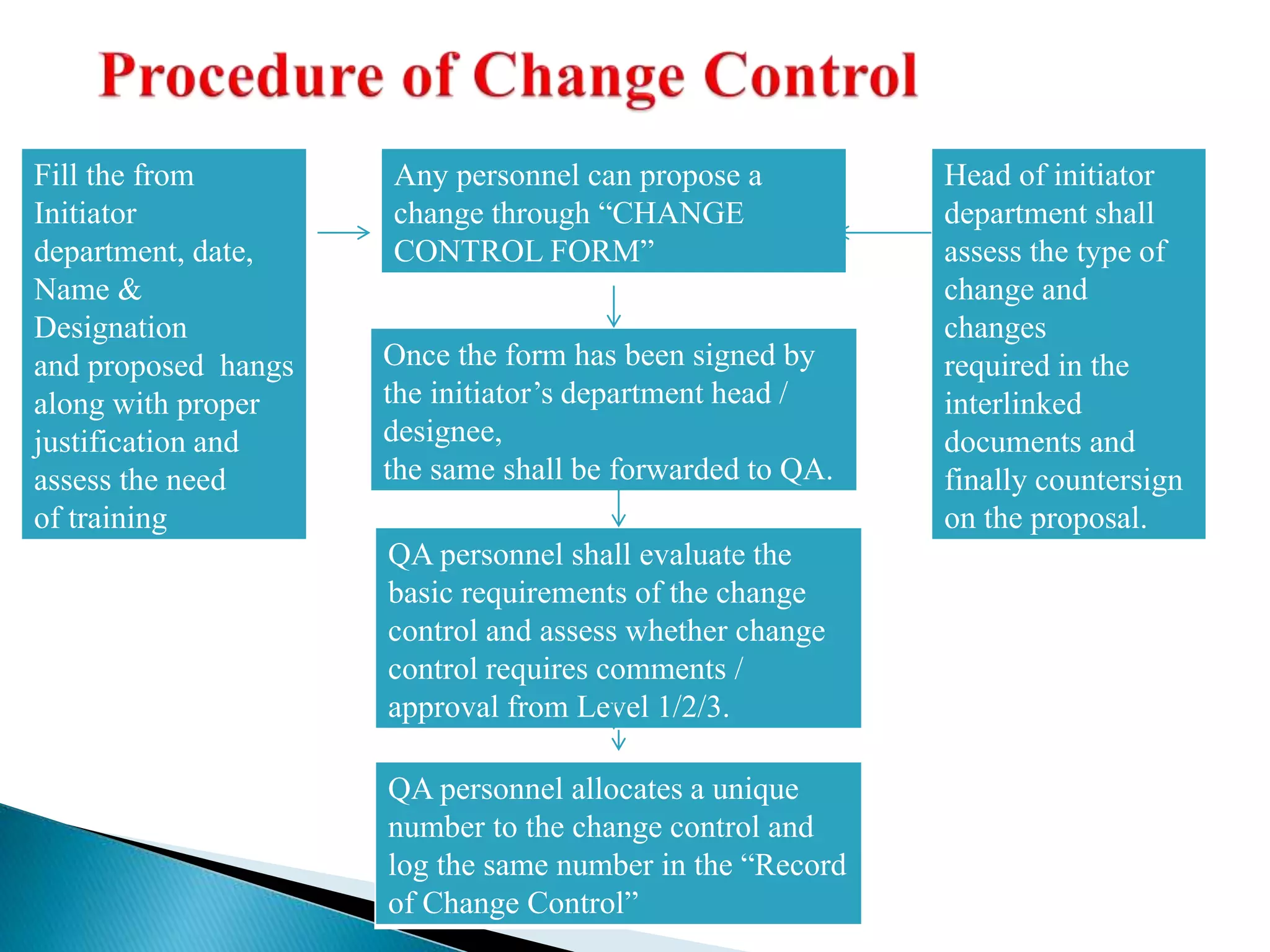
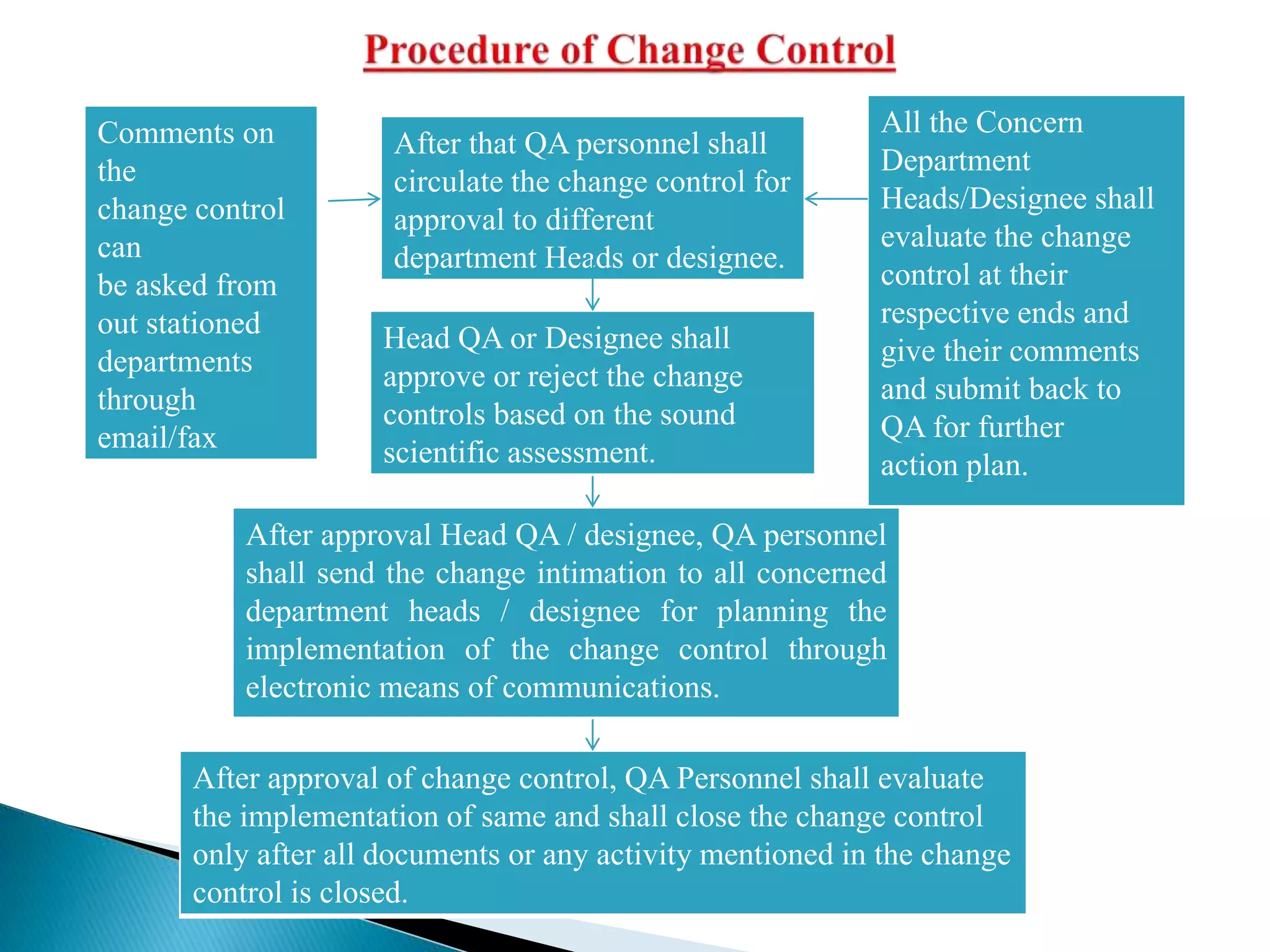
The document discusses calculation of yields, production record review, and change control in the pharmaceutical industry. It provides definitions and requirements for theoretical yield, actual yield, and practical yield calculation. It states that all production records must be reviewed and approved before batch release. Any unexplained discrepancies or failed batches must be investigated. The document also defines minor, major, and critical changes and the proper change control process, including documenting the request, assessing the change, planning implementation, verifying the impact, implementing, and closing out the change.