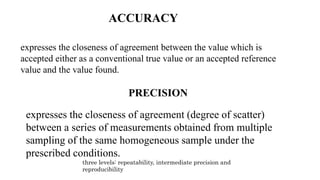
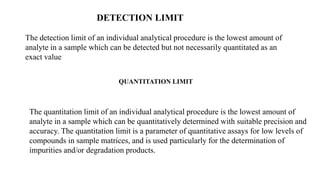
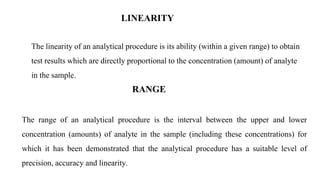
This document discusses validation parameters for analytical methods according to the International Council for Harmonisation guidelines. It lists accuracy, precision (repeatability, intermediate precision), specificity, detection limit, quantitation limit, linearity, range, and robustness as key validation characteristics that should be considered. It provides definitions and details for each validation characteristic. Revalidation may be necessary if there are changes in the drug substance synthesis, product composition, or analytical procedure.