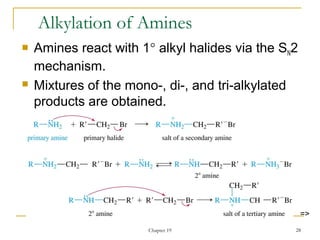
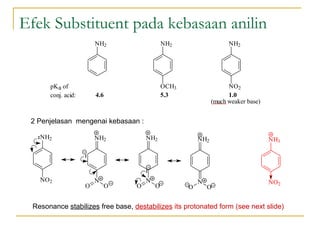
This document discusses various topics related to amines including their nomenclature, properties, reactions, and synthesis. It defines primary, secondary, tertiary, and quaternary amines based on the number of alkyl or aryl groups bonded to the nitrogen atom. It also discusses the basicity of amines, noting that amines are weak bases due to their ability to donate a lone pair of electrons. Substituents on the nitrogen atom such as alkyl groups increase the basicity by stabilizing the conjugate acid. Common reactions of amines include electrophilic substitution, alkylation, acylation, oxidation, and reactions with nitrous acid to form diazonium salts. Amines can also be synthesized through reduct













![Kebassan amina
Amin sedikit basa, karena mempunyai pasangan
elektron bebas dapat mendonorkan ke proton,
bersifat nucleophiles.
Amina mempunyai nilai Kb = 10-3
to 10-4
RNH2 + H OH RNH3 + OH
Kb =
[RNH3 ] [OH ]
[RNH2]](https://image.slidesharecdn.com/aminabaru-150611035625-lva1-app6892/85/Amina-baru-14-320.jpg)
![Kebasaan amina -------
Biasa digunakan nilai Ka asam konjugat amina
Asam konjugat lebih lemah, lebih basa (amina)
Amina sejenis mempunyai Ka values (conjugate
acids) dari 10-10
sampai 10-11
(nilai pKa 10 sampai
11.)
RNH3 RNH2 + H Ka =
[RNH3 ]
[RNH2] [H ]](https://image.slidesharecdn.com/aminabaru-150611035625-lva1-app6892/85/Amina-baru-15-320.jpg)





![Amina terprotonasi pada pH Physiological
Persamaan Henderson-Hasselbalch :
Consider the neurotransmitter dopamine, a typical amine (having a pKa of its
conjugate acid = 10.6) in a living cell (buffered at pH = 7.3):
That is, the concentration of the protonated amine is 2000x that of the
neutral amine! Typical amines are >99.9% protonated at physiological pH.
pH = pKa + log
[RNH2]
[RNH3
+
]
7.3 = 10.6 + log
[RNH2]
[RNH3
+
]
log
[RNH2]
[RNH3
+
]
-3.3 =
[RNH2]
[RNH3
+
]
2 x 10
3
=
;
;
[RNH2]
[RNH3
+
]
5 x 10
- 4
=](https://image.slidesharecdn.com/aminabaru-150611035625-lva1-app6892/85/Amina-baru-22-320.jpg)