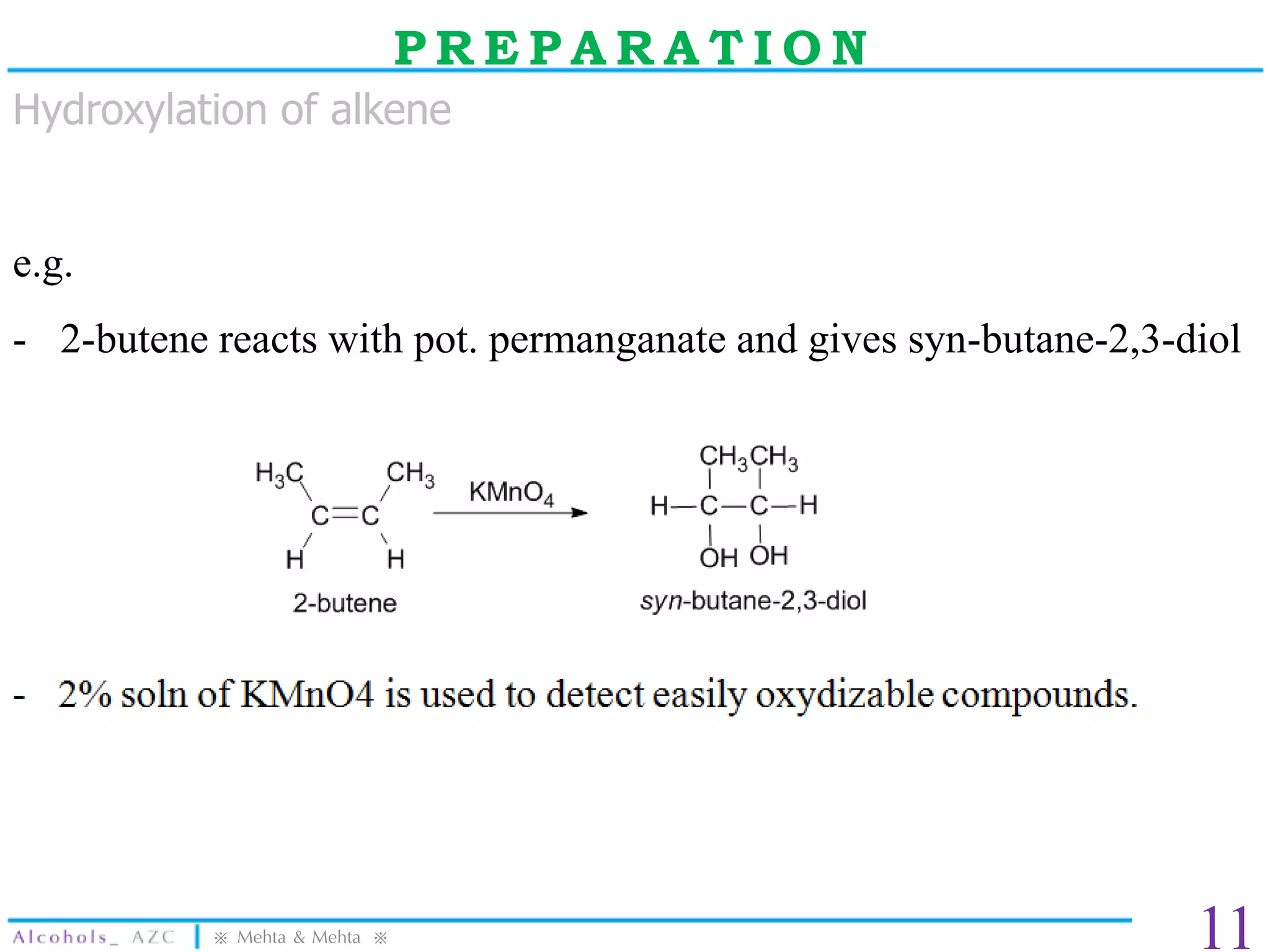
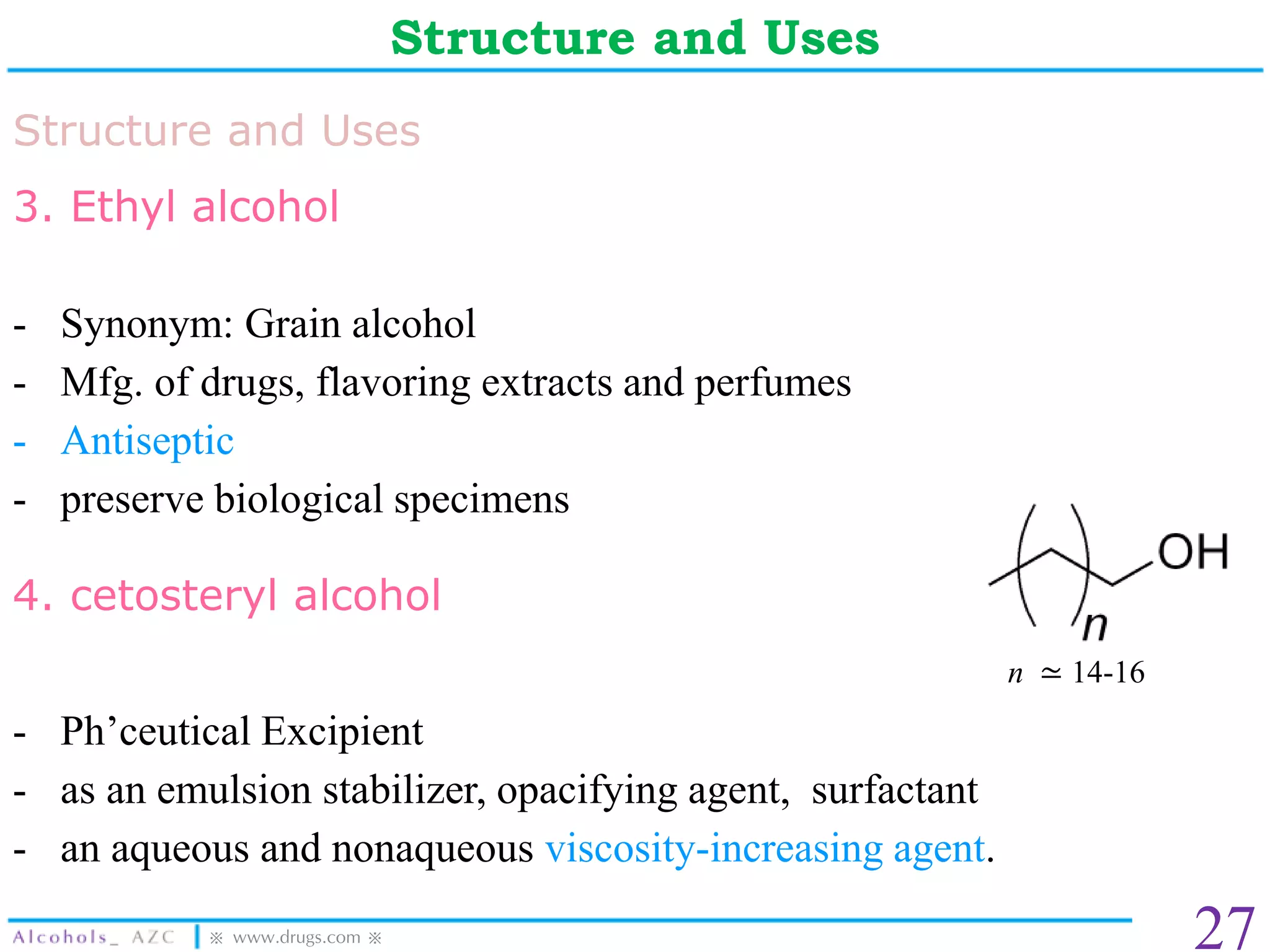
The document provides a comprehensive overview of alcohols, detailing their properties, preparation methods, and reactions. It includes specific boiling points, solubility trends, and various synthesis techniques, such as the use of Grignard reagents and oxidation tests. Additionally, it outlines the uses of several alcohols in pharmaceuticals and other applications.