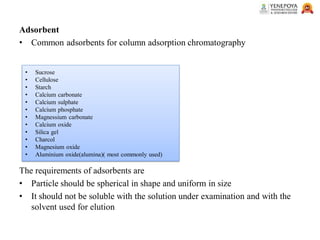
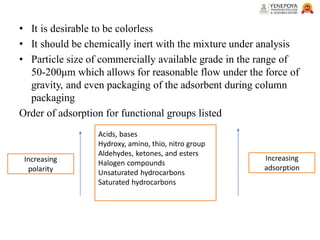
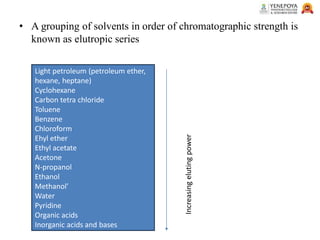
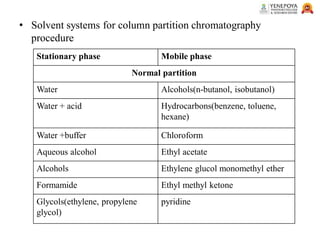
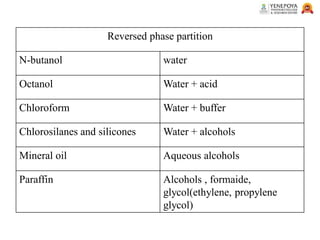
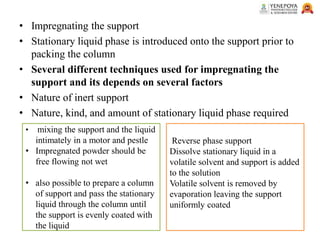
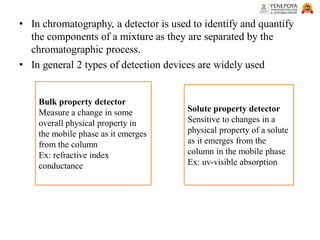
This presentation provides an in-depth overview of chromatography, focusing on adsorption and partition chromatography. It covers the principles, methodologies, types, classification, column preparation, detection methods, advantages, disadvantages, and pharmaceutical applications. A useful resource for pharmacy students and professionals in pharmaceutical chemistry.