1. This document provides an overview of thin layer chromatography (TLC) including adsorbents, preparation techniques, mobile phase selection, and reverse phase TLC.
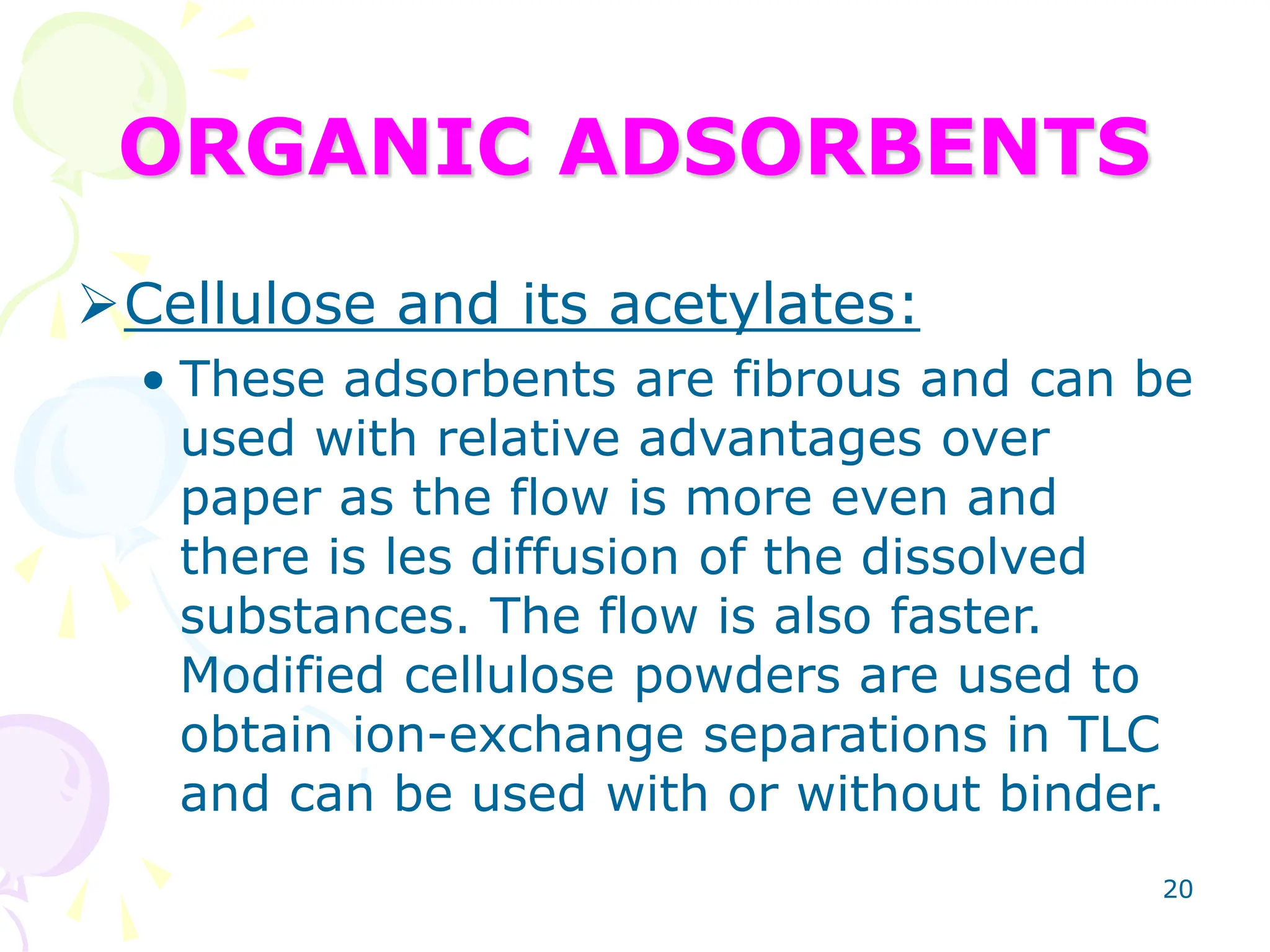
2. Common adsorbents for TLC plates include silica gel, alumina, and cellulose. Factors like particle size and chemical properties are considered when selecting an adsorbent.
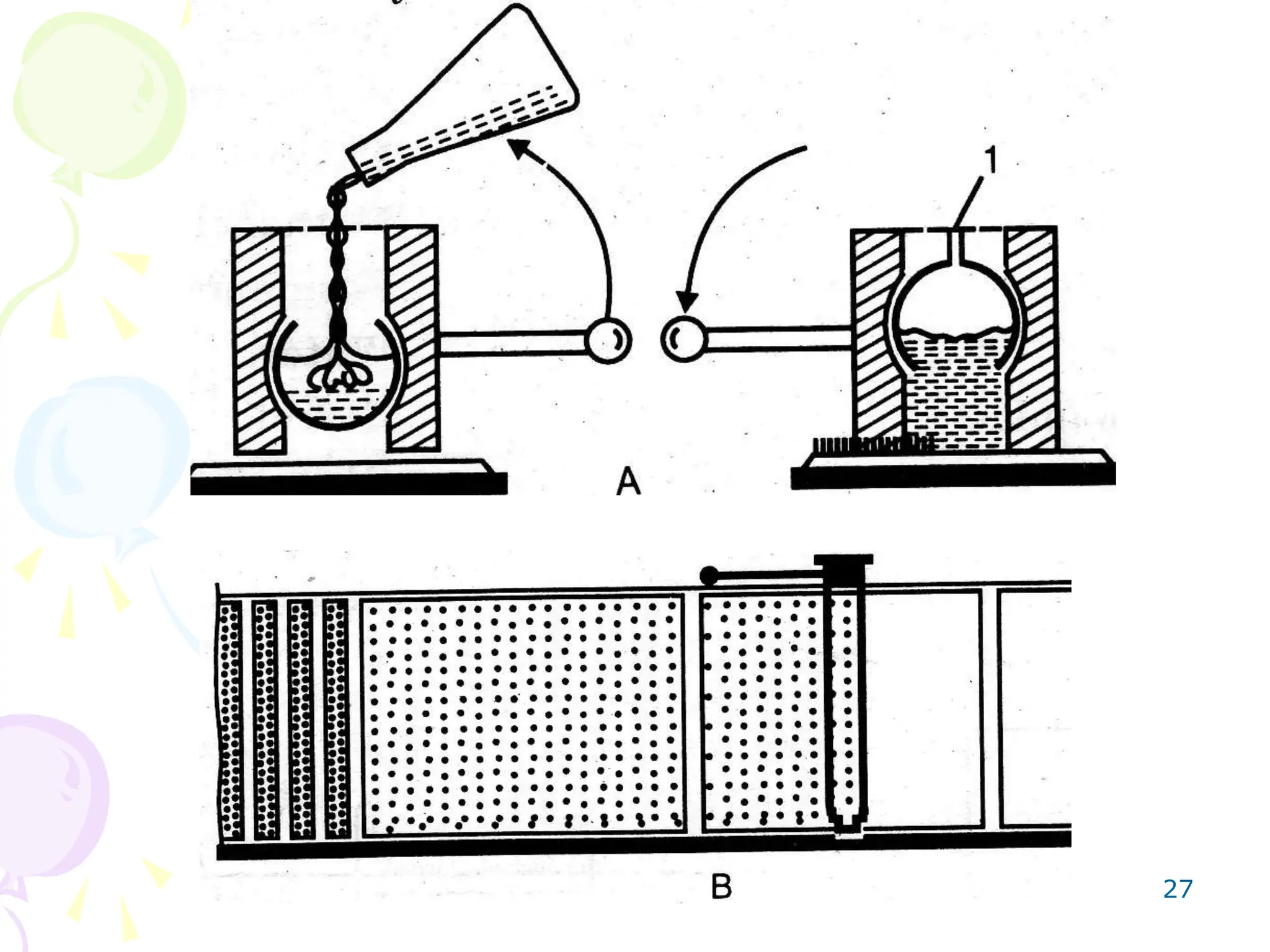
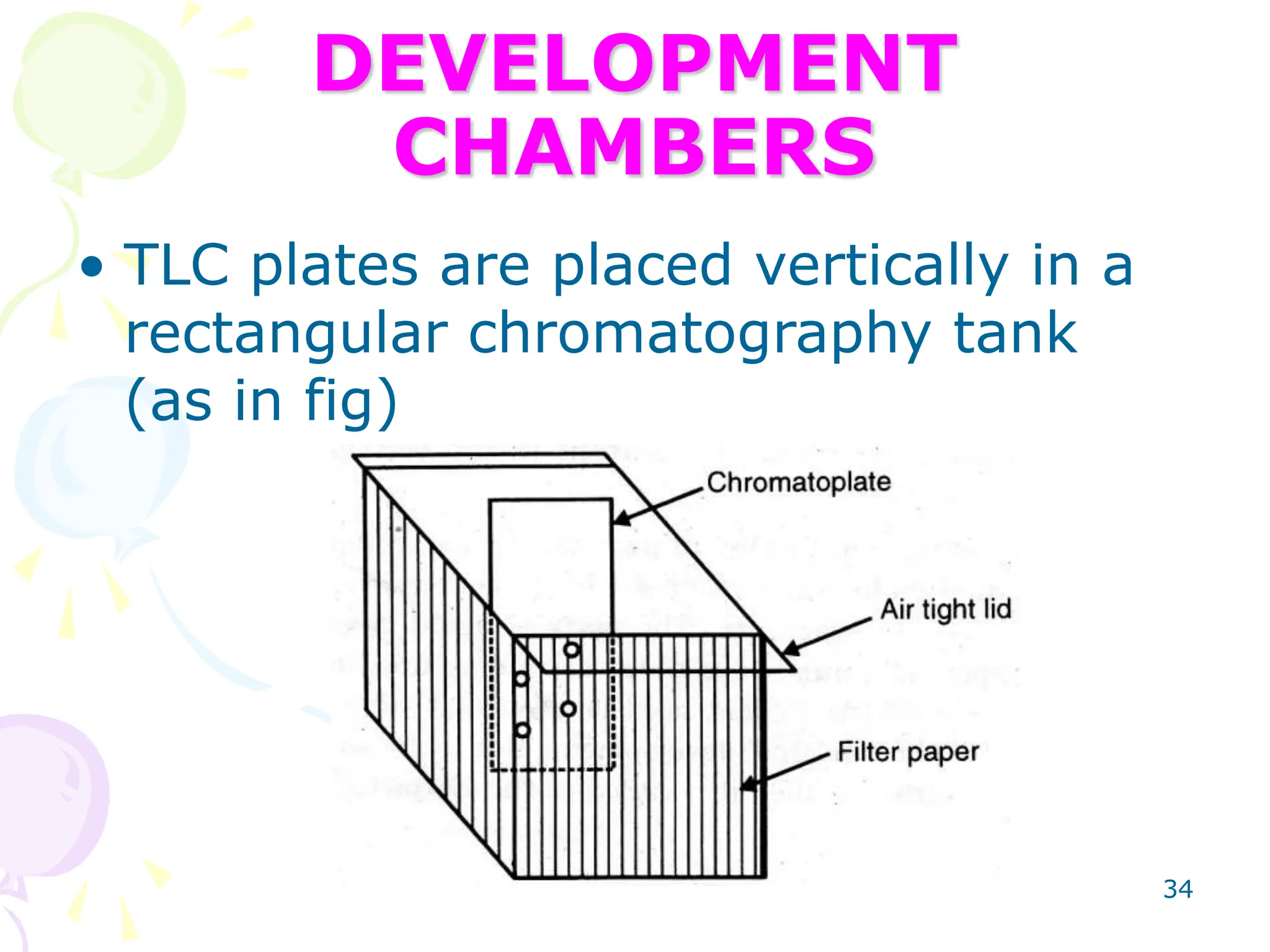
3. TLC plates are prepared by coating glass or plastic plates with a thin, uniform layer of adsorbent using various techniques like pouring, dipping, spraying, or spreading.
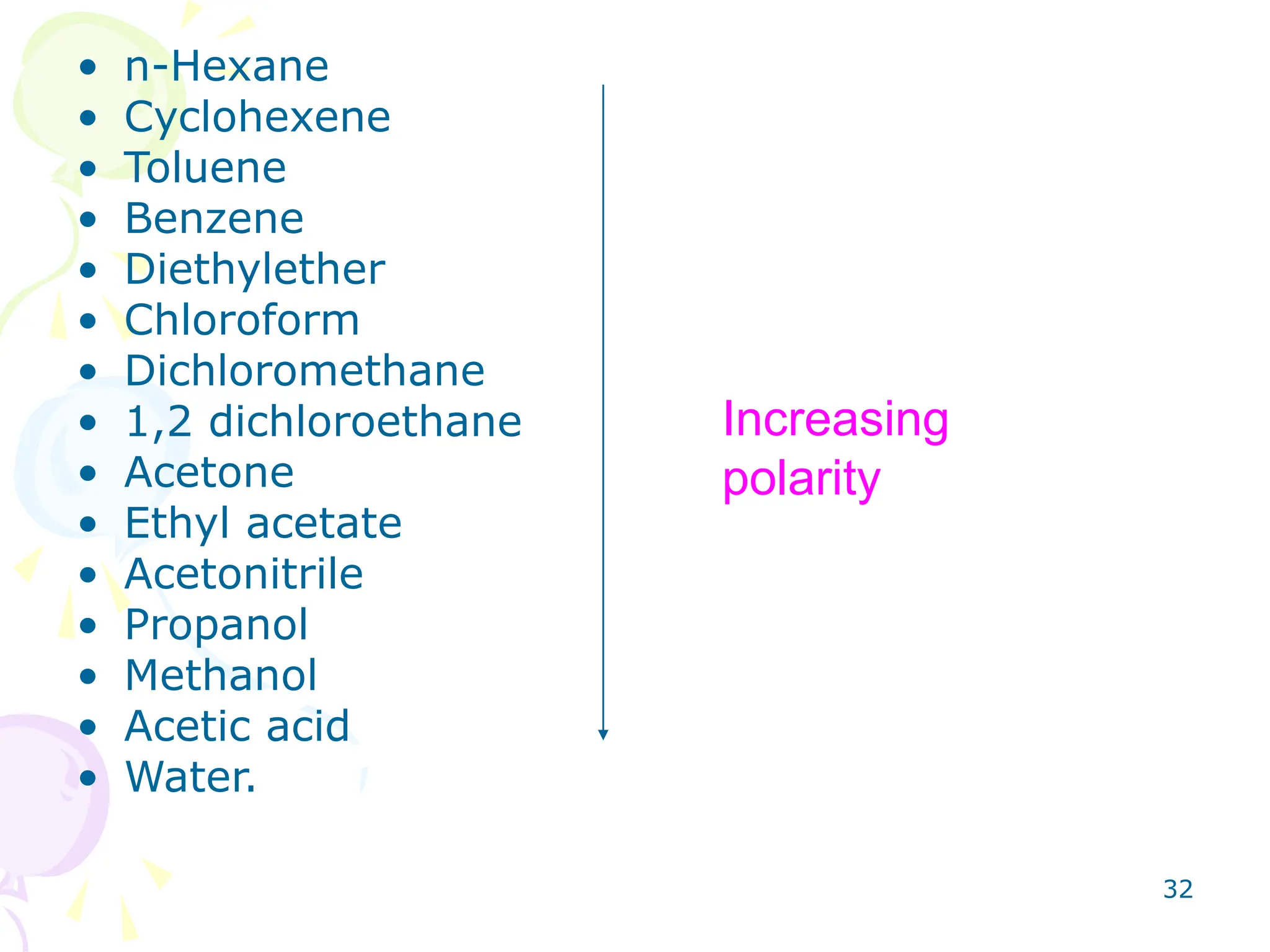
4. Mobile phase selection depends on factors like the compounds being separated and the adsorbent used. Mobile phases range from non-