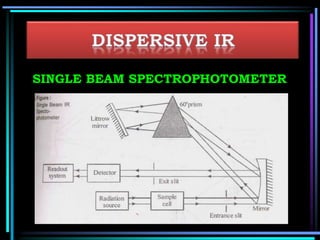
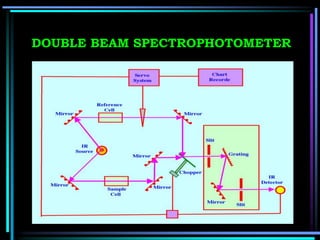
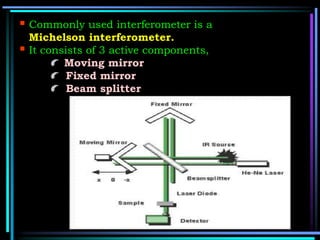
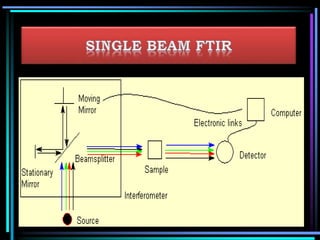
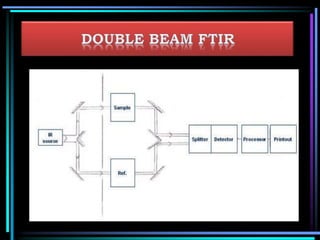
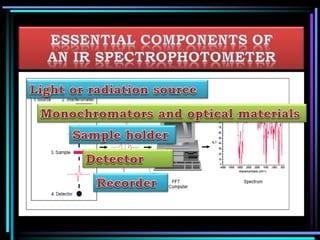
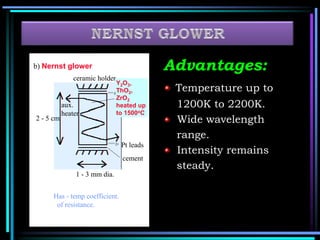
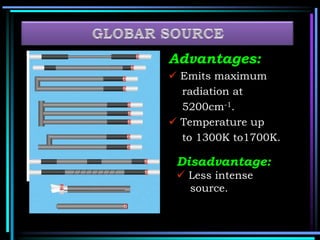
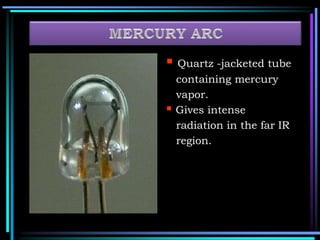
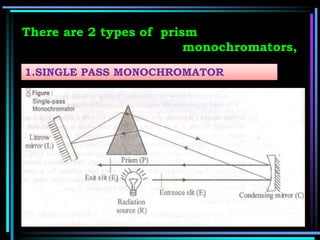
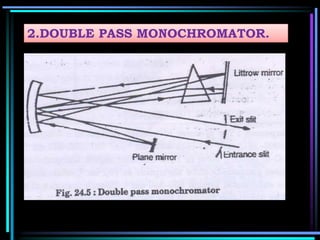
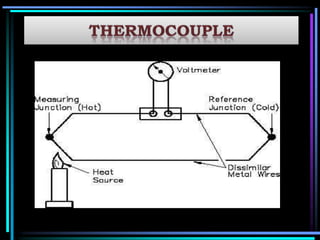
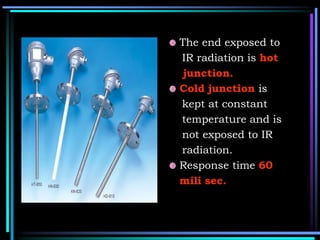
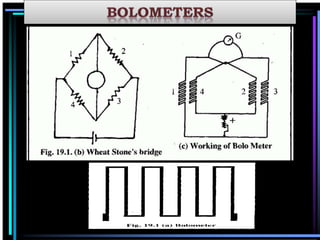
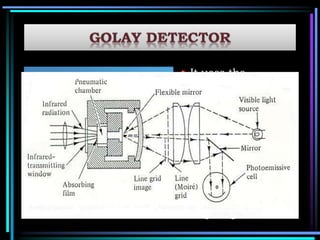
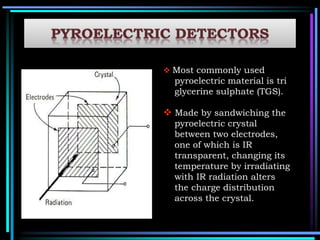
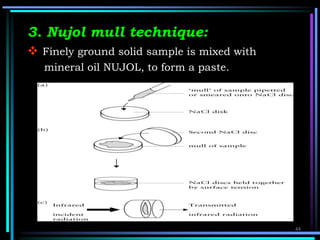
This document discusses different types of infrared spectrophotometers and their components. It describes three main types - dispersive, Fourier transform, and non-dispersive spectrophotometers. Fourier transform spectrometers use an interferometer, typically a Michelson interferometer, along with a source, detector, and moving mirror to obtain spectra. The document also outlines various infrared radiation sources, monochromators, detectors, sampling techniques and gas cells used in infrared spectrophotometry.