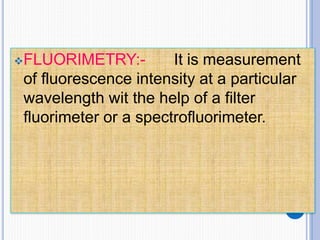
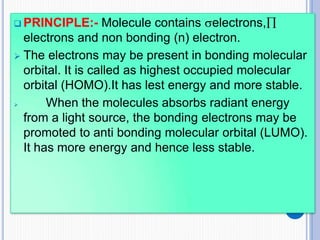
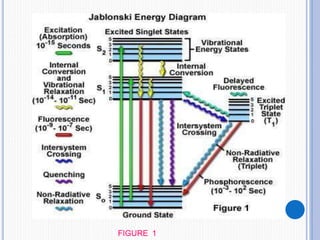
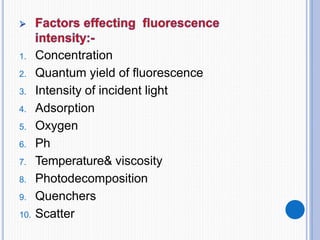
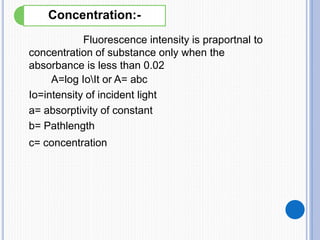
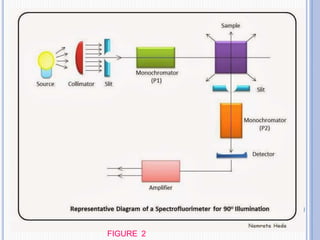
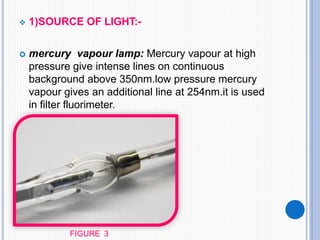
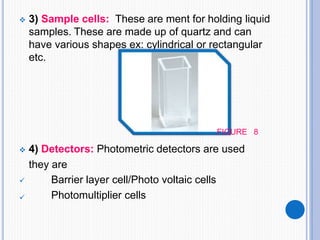
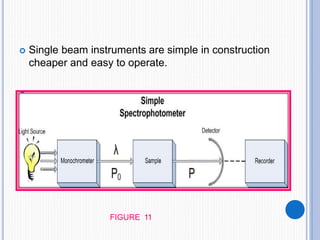
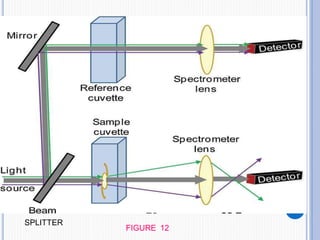
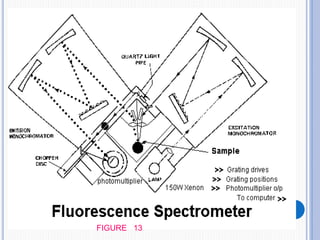
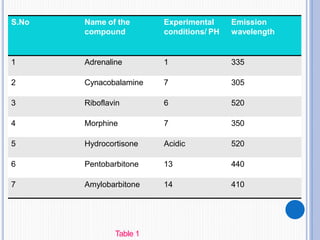
The document discusses fluorescence, including its principles, measurement through fluorimetry, and factors affecting fluorescence intensity. It covers the processes involved in fluorescence and phosphorescence, various types of quenching, and specific instrumentation used for fluorescence measurements such as light sources, filters, and detectors. Additionally, it details applications of fluorimetry in analyzing various substances, emphasizing its advantages in quantitative analysis and sensitivity.