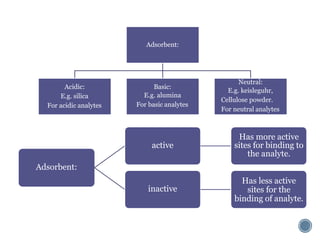
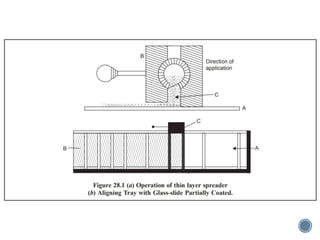
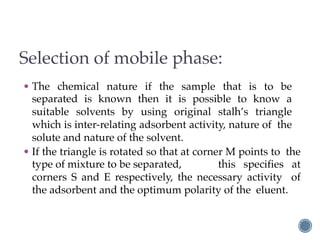
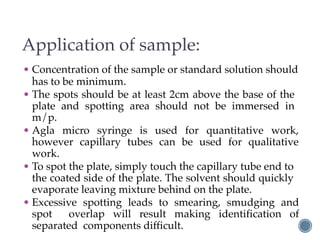
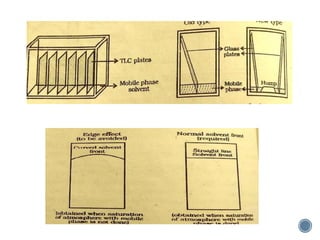
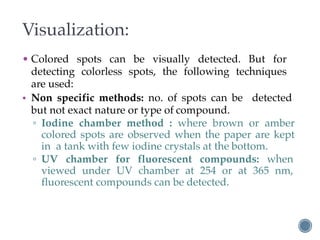
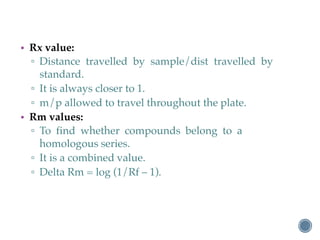
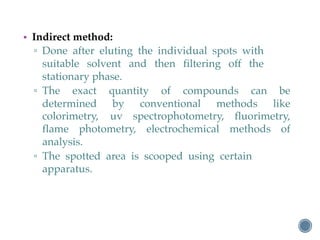
Thin layer chromatography is a technique used to separate mixtures based on differences in how compounds partition between a stationary phase and a mobile phase. It involves coating a thin layer of adsorbent on a plate, spotting samples, and developing the plate in a mobile phase. Compounds separate based on their affinity for the stationary versus mobile phase. Rf values characterize separation and allow identification of unknowns. TLC is useful for analyzing organic compounds, drug purity testing, and reaction monitoring due to its low cost, simplicity, and speed.