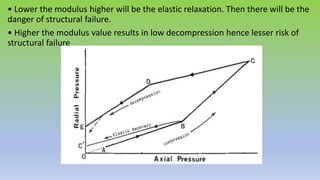
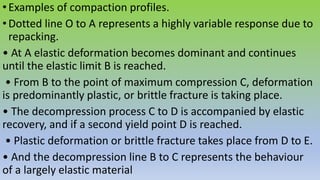
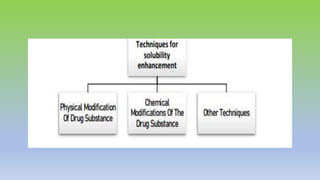
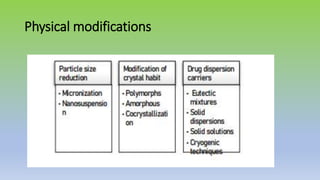
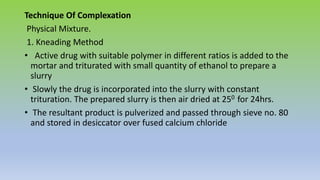
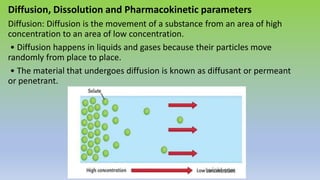
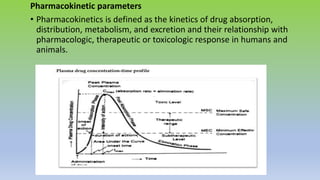
The document explores various techniques for enhancing drug solubility and discusses the significance of compaction profiles in drug formulation. Key topics include particle size reduction through micronization and nanosuspension, as well as methods like cocrystallization and solid dispersions to improve bioavailability of poorly water-soluble drugs. It emphasizes the importance of solubility in pharmaceutical development, detailing physical and chemical modification techniques to facilitate drug absorption and efficacy.