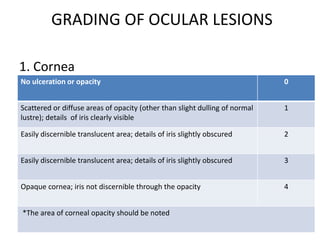
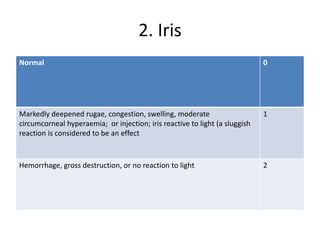
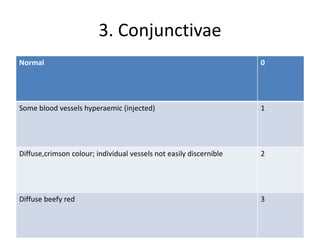
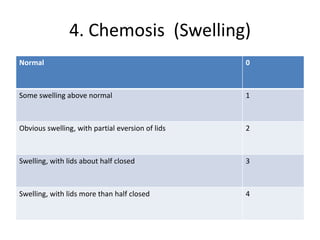
The document outlines OECD Guideline 405 for testing acute eye irritation and corrosion, emphasizing animal welfare by promoting the use of analgesics and anesthetics to reduce pain during in vivo testing. The protocol includes recommendations for initial and confirmatory tests, housing conditions, administration of test substances, monitoring of animal welfare, and thorough data reporting to ensure ethical treatment of test subjects. It underscores the importance of evaluating reversibility of effects and humane endpoints for animal euthanasia to prevent unnecessary suffering.