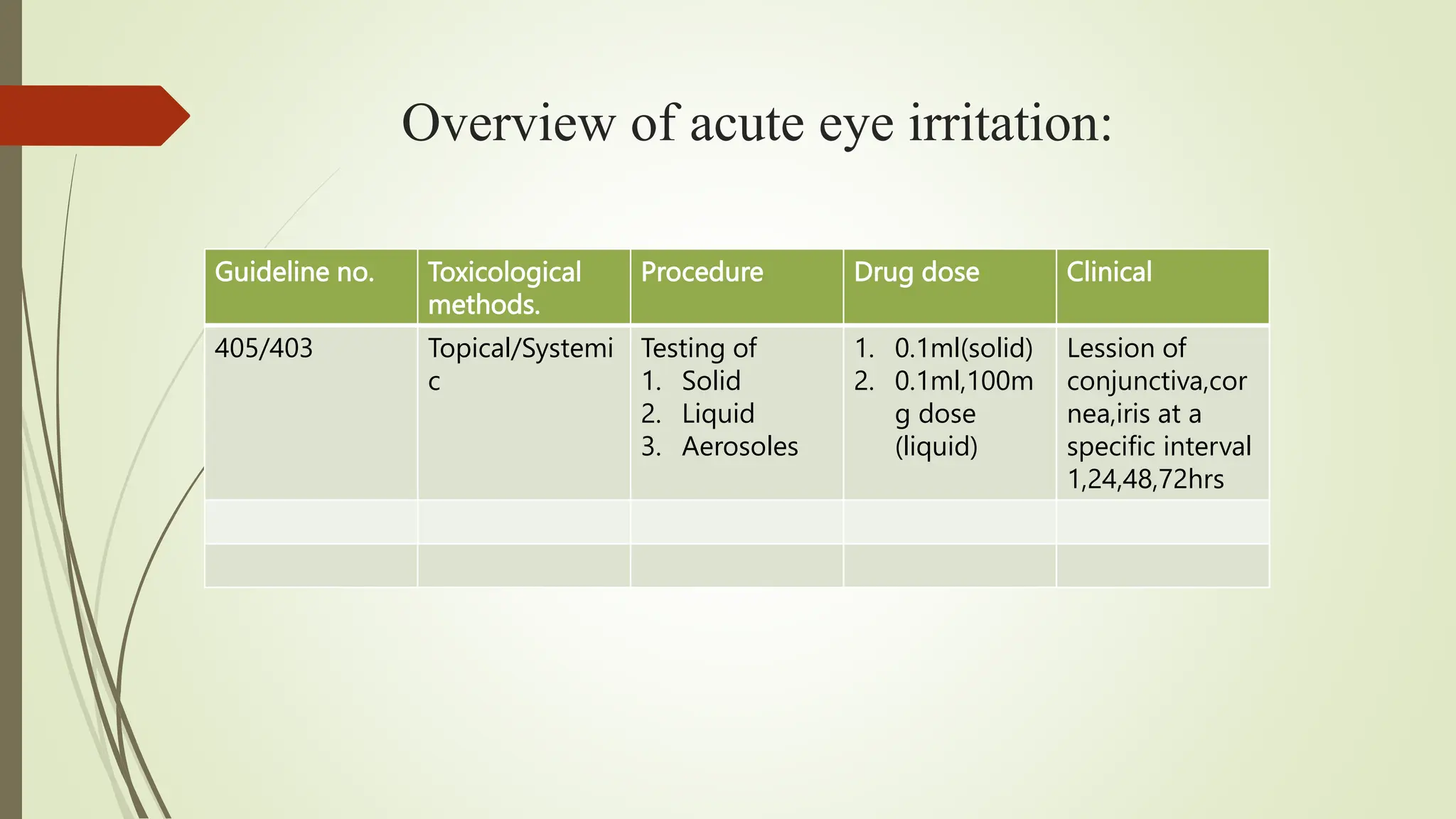
The document outlines the OECD Guideline 405 for acute eye irritation testing, emphasizing the evaluation of ocular toxicity through structured procedures and animal welfare considerations. It details the classification of irritants, the protocol for initial and confirmatory tests using laboratory animals, and recommendations for analgesia management during the testing process. The guideline aims to minimize unnecessary animal testing while ensuring accurate and reliable toxicity assessments of chemicals.