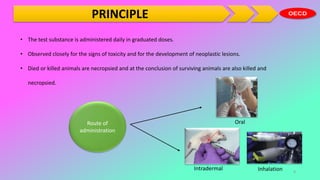
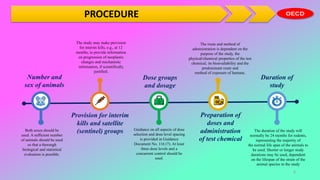
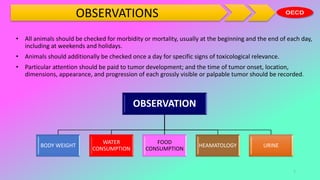
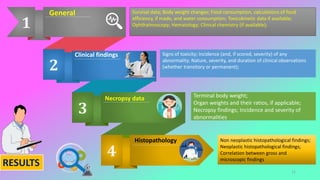
This document outlines the OECD guidelines for carcinogenicity testing. It discusses the objectives of carcinogenicity studies which include identifying carcinogenic properties, target organs, time to appearance of neoplasms, and establishing a no-observed-adverse-effect level. It describes the typical procedure which involves administering the test substance daily to rodents and observing them for signs of toxicity and tumor development. Key aspects covered are test animals, dose levels, duration of study, observations, pathology examination, and reporting of results and test conditions.