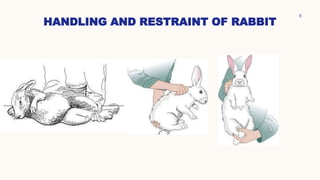
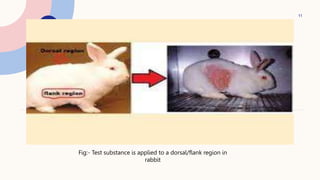
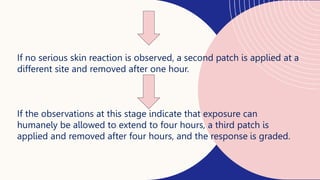
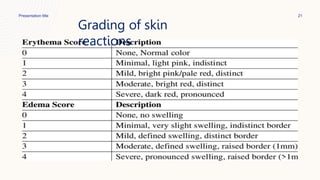
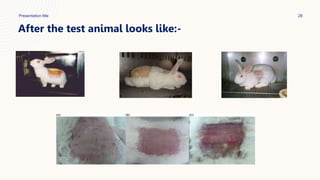
This document summarizes dermal irritation and dermal toxicity studies. It discusses the principles, procedures, observations, and grading of dermal irritation studies using rabbits. Key steps include applying a test chemical to rabbit skin for 4 hours and observing any irritation at time points post-application. It also summarizes the principles, test procedures, requirements, and observations of dermal toxicity studies in rabbits, which involve applying various doses of a test substance to shaved skin and observing local and systemic effects.