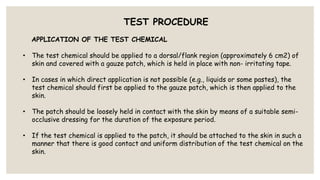
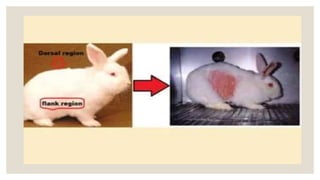
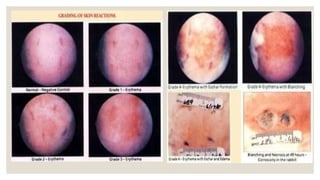
This document summarizes a dermal irritation study conducted according to OECD guidelines using rabbits. It describes procedures for handling, restraining, and housing the rabbits, as well as applying the test chemical to their skin for 4 hours. Observations are made at several time points over 14 days to evaluate the degree of irritation or corrosion and reversibility of any effects. Scoring is based on criteria for erythema, edema, and other reactions. The study aims to safely and humanely evaluate the skin toxicity of chemicals.