Embed presentation
Downloaded 133 times






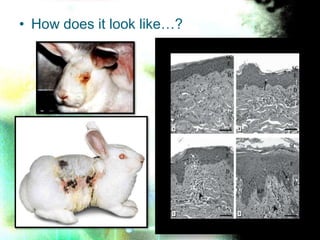



This document discusses acute dermal toxicity testing. It provides an introduction stating that dermal toxicity testing assesses the toxic effects of a substance applied to skin. Key terminology is defined, such as LD50. The test procedure involves applying graduated doses of a test substance to animal skin and observing for signs of toxicity. Animals are clinically examined and necropsied to determine cause of death or signs of distress. Data is reported on treatment results, including LD50 values.









