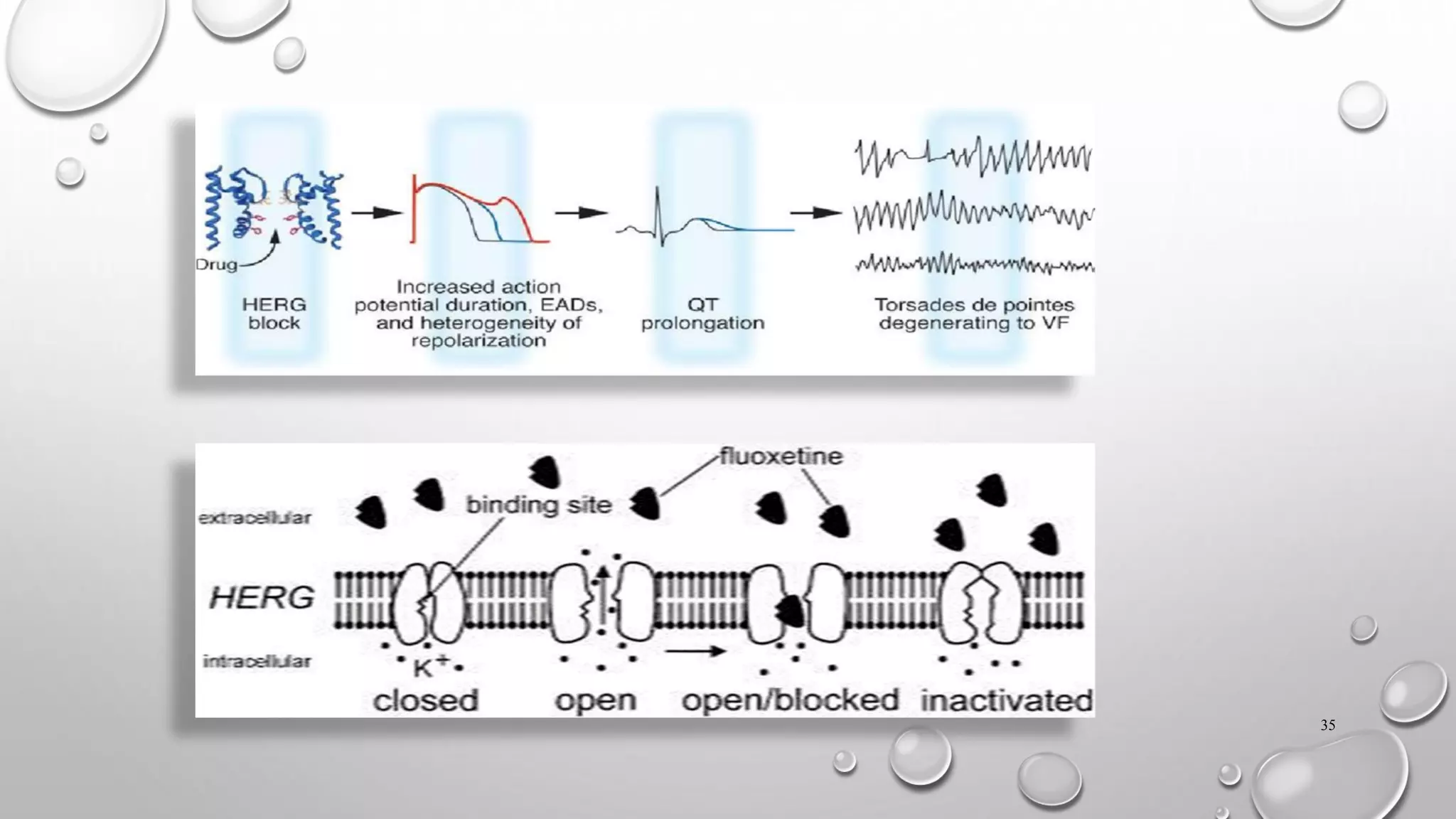
This document discusses the requirements for an investigational new drug (IND) application. An IND is required to initiate clinical trials of an unapproved drug and must contain information on animal studies, manufacturing, and clinical trial protocols. The core battery of safety pharmacology studies evaluates effects on major organ systems like the cardiovascular, central nervous, and respiratory systems. These studies are designed to identify potential adverse effects and safety risks before human clinical trials.